 | |
| Names | |
|---|---|
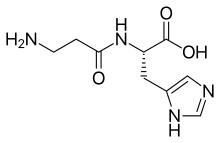
| IUPAC name
β-Alanylhistidine | |
| Systematic IUPAC name
(2S)-2-(3-Aminopropanamido)-3-(3H-imidazol-4-yl)propanoic acid | |
| Other names
β-Alanyl-L-histidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.610 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H14N4O3 | |
| Molar mass | 226.236 g·mol−1 |
| Appearance | Crystalline solid |
| Melting point | 253 °C (487 °F; 526 K) (decomposition) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Carnosine (beta-alanyl-L-histidine) is a dipeptide molecule, made up of the amino acids beta-alanine and histidine. It is highly concentrated in muscle and brain tissues. Carnosine was discovered by Russian chemist Vladimir Gulevich.[1]
Carnosine is naturally produced by the body in the liver[2] from beta-alanine and histidine. Like carnitine, carnosine is composed of the root word carn, meaning "flesh", alluding to its prevalence in meat.[3] There are no plant-based sources of carnosine.[4] Carnosine is readily available as a synthetic nutritional supplement.
Biosynthesis
Carnosine is synthesized within the body from beta-alanine and histidine. Beta-alanine is a product of pyrimidine catabolism[5] and histidine is an essential amino acid. Since beta-alanine is the limiting substrate, supplementing just beta-alanine effectively increases the intramuscular concentration of carnosine.[6][7]
Physiological effects
pH buffer
Carnosine has a pKa value of 6.83, making it a good buffer for the pH range of animal muscles.[8] Since beta-alanine is not incorporated into proteins, carnosine can be stored at relatively high concentrations (millimolar). Occurring at 17–25 mmol/kg (dry muscle),[9] carnosine (β-alanyl-L-histidine) is an important intramuscular buffer, constituting 10-20% of the total buffering capacity in type I and II muscle fibres.
Anti-oxidant
Carnosine has been shown to scavenge reactive oxygen species (ROS) as well as alpha-beta unsaturated aldehydes formed from peroxidation of cell membrane fatty acids during oxidative stress. It also buffers pH in muscle cells, and acts as a neurotransmitter in the brain. It is also a zwitterion, a neutral molecule with a positive and negative end.
Antiglycating
Carnosine acts as an antiglycating agent, reducing the rate of formation of advanced glycation end-products (substances that can be a factor in the development or worsening of many degenerative diseases, such as diabetes, atherosclerosis, chronic kidney failure, and Alzheimer's disease[10]), and ultimately reducing development of atherosclerotic plaque build-up.[11][12][13]
Geroprotective
Carnosine is considered as a geroprotector.[14] Carnosine can increase the Hayflick limit in human fibroblasts,[15] as well as appearing to reduce the telomere shortening rate.[16] Carnosine may also slow aging through its anti-glycating properties (chronic glycolyating is speculated to accelerate aging).[17]
Other
Carnosine can chelate divalent metal ions.[11][18] It has been suggested that binding Ca2+ may displace protons, thereby providing a link between Ca2+ and H+ buffering. [19] However, there is still controversy as to how much Ca2+ is bound to carnosine under physiological conditions. [20]
Research has demonstrated a positive association between muscle tissue carnosine concentration and exercise performance.[21][22][23] β-Alanine supplementation is thought to increase exercise performance by promoting carnosine production in muscle. Exercise has conversely been found to increase muscle carnosine concentrations, and muscle carnosine content is higher in athletes engaging in anaerobic exercise.[21]
See also
- Acetylcarnosine, a similar molecule used to treat lens cataracts
- Anserine, another dipeptide antioxidant (found in birds)
- Carnosine synthase, an enzyme that helps carnosine production
- Carnosinemia, a disease of excess carnosine due to an enzyme defect/deficiency
References
- ↑ Gulewitsch, Wl.; Amiradžibi, S. (1900). "Ueber das Carnosin, eine neue organische Base des Fleischextractes". Berichte der Deutschen Chemischen Gesellschaft. 33 (2): 1902–1903. doi:10.1002/cber.19000330275.
- ↑ Trexler, Eric T.; Smith-Ryan, Abbie E.; Stout, Jeffrey R.; Hoffman, Jay R.; Wilborn, Colin D.; Sale, Craig; Kreider, Richard B.; Jäger, Ralf; Earnest, Conrad P.; Bannock, Laurent; Campbell, Bill (2015-07-15). "International society of sports nutrition position stand: Beta-Alanine". Journal of the International Society of Sports Nutrition. 12: 30. doi:10.1186/s12970-015-0090-y. ISSN 1550-2783. PMC 4501114. PMID 26175657.
- ↑ Hipkiss, A. R. (2006). "Does chronic glycolysis accelerate aging? Could this explain how dietary restriction works?". Annals of the New York Academy of Sciences. 1067 (1): 361–8. Bibcode:2006NYASA1067..361H. doi:10.1196/annals.1354.051. PMID 16804012. S2CID 41175541.
- ↑ Alan R. Hipkiss (2009). "Chapter 3: Carnosine and Its Possible Roles in Nutrition and Health". Advances in Food and Nutrition Research.
- ↑ "beta-ureidopropionate + H2O => beta-alanine + NH4+ + CO2". reactome. Archived from the original on 2013-10-23. Retrieved 2020-02-08.
Cytosolic 3-ureidopropionase catalyzes the reaction of 3-ureidopropionate and water to form beta-alanine, CO2, and NH3 (van Kuilenberg et al. 2004).
- ↑ Derave W, Ozdemir MS, Harris R, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E (August 9, 2007). "Beta-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters". J Appl Physiol. 103 (5): 1736–43. doi:10.1152/japplphysiol.00397.2007. PMID 17690198. S2CID 6990201.
- ↑ Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007). "Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity". Amino Acids. 32 (2): 225–33. doi:10.1007/s00726-006-0364-4. PMID 16868650. S2CID 23988054.
- ↑ Bate-Smith, EC (1938). "The buffering of muscle in rigor: protein, phosphate and carnosine". Journal of Physiology. 92 (3): 336–343. doi:10.1113/jphysiol.1938.sp003605. PMC 1395289. PMID 16994977.
- ↑ Mannion, AF; Jakeman, PM; Dunnett, M; Harris, RC; Willan, PLT (1992). "Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans". Eur. J. Appl. Physiol. 64 (1): 47–50. doi:10.1007/BF00376439. PMID 1735411. S2CID 24590951.
- ↑ Vistoli, G; De Maddis, D; Cipak, A; Zarkovic, N; Carini, M; Aldini, G (Aug 2013). "Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation". Free Radic. Res. 47: Suppl 1:3–27. doi:10.3109/10715762.2013.815348. PMID 23767955. S2CID 207517855.
- 1 2 Reddy, V. P.; Garrett, MR; Perry, G; Smith, MA (2005). "Carnosine: A Versatile Antioxidant and Antiglycating Agent". Science of Aging Knowledge Environment. 2005 (18): pe12. doi:10.1126/sageke.2005.18.pe12. PMID 15872311.
- ↑ Rashid, Imran; Van Reyk, David M.; Davies, Michael J. (2007). "Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro". FEBS Letters. 581 (5): 1067–70. doi:10.1016/j.febslet.2007.01.082. PMID 17316626. S2CID 46535145.
- ↑ Hipkiss, A. R. (2005). "Glycation, ageing and carnosine: Are carnivorous diets beneficial?". Mechanisms of Ageing and Development. 126 (10): 1034–9. doi:10.1016/j.mad.2005.05.002. PMID 15955546. S2CID 19979631.
- ↑ Boldyrev, A. A.; Stvolinsky, S. L.; Fedorova, T. N.; Suslina, Z. A. (2010). "Carnosine as a natural antioxidant and geroprotector: From molecular mechanisms to clinical trials". Rejuvenation Research. 13 (2–3): 156–8. doi:10.1089/rej.2009.0923. PMID 20017611.
- ↑ McFarland, G; Holliday, R (1994). "Retardation of the Senescence of Cultured Human Diploid Fibroblasts by Carnosine". Experimental Cell Research. 212 (2): 167–75. doi:10.1006/excr.1994.1132. PMID 8187813.
- ↑ Shao, Lan; Li, Qing-Huan; Tan, Zheng (2004). "L-Carnosine reduces telomere damage and shortening rate in cultured normal fibroblasts". Biochemical and Biophysical Research Communications. 324 (2): 931–6. doi:10.1016/j.bbrc.2004.09.136. PMID 15474517.
- ↑ Hipkiss, A. R. (2006). "Does Chronic Glycolysis Accelerate Aging? Could This Explain How Dietary Restriction Works?". Annals of the New York Academy of Sciences. 1067 (1): 361–8. Bibcode:2006NYASA1067..361H. doi:10.1196/annals.1354.051. PMID 16804012. S2CID 41175541.
- ↑ Abate, Chiara; Cassone, Giuseppe; Cordaro, Massimiliano; Giuffrè, Ottavia; Mollica-Nardo, Viviana; Ponterio, Rosina Celeste; Saija, Franz; Sponer, Jiri; Trusso, Sebastiano; Foti, Claudia (2021). "Understanding the behaviour of carnosine in aqueous solution: an experimental and quantum-based computational investigation on acid–base properties and complexation mechanisms with Ca 2+ and Mg 2+". New Journal of Chemistry. 45 (43): 20352–20364. doi:10.1039/D1NJ04094D. ISSN 1144-0546.
- ↑ Swietach, Pawel; Youm, Jae-Boum; Saegusa, Noriko; Leem, Chae-Hun; Spitzer, Kenneth W.; Vaughan-Jones, Richard D. (2013-05-28). "Coupled Ca2+/H+ transport by cytoplasmic buffers regulates local Ca2+ and H+ ion signaling". Proceedings of the National Academy of Sciences of the United States of America. 110 (22): E2064–2073. doi:10.1073/pnas.1222433110. ISSN 1091-6490. PMC 3670334. PMID 23676270.
- ↑ Eisner, David; Neher, Erwin; Taschenberger, Holger; Smith, Godfrey (2023-06-16). "Physiology of intracellular calcium buffering". Physiological Reviews. 103 (4): 2767–2845. doi:10.1152/physrev.00042.2022. ISSN 1522-1210. PMID 37326298.
- 1 2 Culbertson, Julie Y.; Kreider, Richard B.; Greenwood, Mike; Cooke, Matthew (2010-01-25). "Effects of Beta-Alanine on Muscle Carnosine and Exercise Performance:A Review of the Current Literature". Nutrients. 2 (1): 75–98. doi:10.3390/nu2010075. ISSN 2072-6643. PMC 3257613. PMID 22253993.
- ↑ Baguet, Audrey; Bourgois, Jan; Vanhee, Lander; Achten, Eric; Derave, Wim (2010-07-29). "Important role of muscle carnosine in rowing performance". Journal of Applied Physiology. 109 (4): 1096–1101. doi:10.1152/japplphysiol.00141.2010. ISSN 8750-7587. PMID 20671038. S2CID 199729.
- ↑ Varanoske, Alyssa N.; Hoffman, Jay R.; Church, David D.; Wang, Ran; Baker, Kayla M.; Dodd, Sarah J.; Coker, Nicholas A.; Oliveira, Leonardo P.; Dawson, Virgil L.; Fukuda, David H.; Stout, Jeffrey R. (2017-09-07). "Influence of Skeletal Muscle Carnosine Content on Fatigue during Repeated Resistance Exercise in Recreationally Active Women". Nutrients. 9 (9): 988. doi:10.3390/nu9090988. ISSN 2072-6643. PMC 5622748. PMID 28880219.