| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
disulfurous acid[1] | |||
| Other names
pyrosulfurous acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
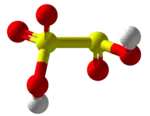
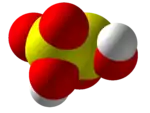
| H2S2O5 | |||
| Molar mass | 146.14 g mol−1 | ||
| Conjugate base | Disulfite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Disulfurous acid or pyrosulfurous acid is an oxoacid of sulfur with the formula H2S2O5. The salts of disulfurous acid are called disulfites or metabisulfites. Disulfurous acid is, like sulfurous acid (H2SO3), a phantom acid, which does not exist in the free state.[2] In contrast to disulfate (S
2O2−
7), disulfite has two directly connected sulfur atoms. The oxidation state of the sulfur atom bonded to three oxygen atoms is +5 while that of the other is +3.
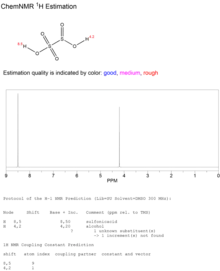
Disulfurous acid 1H NMR spectrum.
References
- ↑ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- ↑ Holleman, Wiberg (2001). Inorganic Chemistry. Academic Press. pp. 537–540. ISBN 9780123526519.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.