 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ferrocenium tetrafluoroborate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.156.161 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C10H10BFeF4 | |
| Molar mass | 272.84 g/mol |
| Appearance | dark blue powder |
| Melting point | 178 °C (352 °F; 451 K) (decomposes) |
| Solubility in acetonitrile | Soluble |
| Hazards[1] | |
| GHS labelling: | |
 | |
| Danger | |
| H314 | |
| P280, P305+P351+P338, P310 | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds |
Ferrocene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
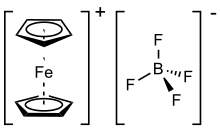
Ferrocenium tetrafluoroborate is an organometallic compound with the formula [Fe(C5H5)2]BF4. This salt is composed of the cation [Fe(C5H5)2]+ and the tetrafluoroborate anion (BF−
4). The related hexafluorophosphate is also a popular reagent with similar properties. The ferrocenium cation is often abbreviated Fc+ or Cp2Fe+. The salt is deep blue in color and paramagnetic.
Ferrocenium salts are sometimes used as one-electron oxidizing agents, and the reduced product, ferrocene, is inert and readily separated from ionic products. The ferrocene–ferrocenium couple is often used as a reference in electrochemistry. The standard potential of ferrocene-ferrocenium is dependent on specific electrochemical conditions.[2]
Preparation
Commercially available, this compound may be prepared by oxidizing ferrocene typically with ferric salts followed by addition of fluoroboric acid.[2] A variety of other oxidants work well also, such as nitrosyl tetrafluoroborate.[3] Many analogous ferrocenium salts are known.[4]
Structure
According to X-ray crystallography, the structures of the metallocene component of FcBF4 and the parent ferrocene are very similar. The Fe-C distances in the cation are 209.5 pm, about 2% longer than the Fe-C distances in ferrocene. [5]
References
- ↑ "Ferrocenium tetrafluoroborate 482358". Sigma-Aldrich.
- 1 2 Connelly, N. G.; Geiger, W. E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chemical Reviews. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ↑ Nielson, Roger M.; McManis, George E.; Safford, Lance K.; Weaver, Michael J. (1989). "Solvent and electrolyte effects on the kinetics of ferrocenium-ferrocene self-exchange. A reevaluation". J. Phys. Chem. 93 (5): 2152. doi:10.1021/j100342a086.
- ↑ Le Bras, J.; Jiao, H.; Meyer, W. E.; Hampel, F.; Gladysz, J. A. (2000). "Synthesis, Crystal Structure, and Reactions of the 17-Valence-Electron Rhenium Methyl Complex [(η5-C5Me5)Re(NO)(P(4-C6H4CH3)3)(CH3)]+ B(3,5-C
6H
3(CF
3)
2)−
4: Experimental and Computational Bonding Comparisons with 18-Electron Methyl and Methylidene Complexes". J. Organomet. Chem. 616: 54–66. doi:10.1016/S0022-328X(00)00531-3. - ↑ Scholz, Stefan; Scheibitz, Matthias; Schödel, Frauke; Bolte, Michael; Wagner, Matthias; Lerner, Hans-Wolfram (2007). "Difference in Reactivity of Triel Halides EX3 Towards Ferrocene". Inorganica Chimica Acta. 360 (10): 3323–3329. doi:10.1016/j.ica.2007.03.049.