 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
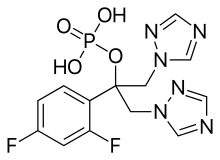
| Formula | C13H13F2N6O4P |
| Molar mass | 386.256 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fosfluconazole (INN) is a water-soluble phosphate prodrug of fluconazole[1] — a triazole antifungal drug used in the treatment and prevention of superficial and systemic fungal infections.[2]
The phosphate ester bond is hydrolyzed by the action of a phosphatase — an enzyme that removes a phosphate group from its substrate by hydrolyzing phosphoric acid monoesters into a phosphate ion and a molecule with a free hydroxyl group (dephosphorylation).[3]
References
- ↑ Bentley A, Butters M, Green SP, Learmonth WJ, MacRae JA, Morland MC, O'Conno G, Skuse J (2002). "The Discovery and Process Development of a Commercial Route to the Water Soluble Prodrug, Fosfluconazole". Organic Process Research & Development. 6 (2): 109–112. doi:10.1021/op010064+. ISSN 1083-6160.
- ↑ Takahashi D, Nakamura T, Shigematsu R, Matsui M, Araki S, Kubo K, et al. (2009-05-25). "Fosfluconazole for antifungal prophylaxis in very low birth weight infants". International Journal of Pediatrics. 2009 (2009): 274768. doi:10.1155/2009/274768. PMC 2778452. PMID 19946419.
- ↑ Lee CC, Schrier WH, Nagyvary J (January 1979). "The enzymatic hydrolysis of the phosphate ester bond in some thionucleotides". Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis. 561 (1): 223–231. doi:10.1016/0005-2787(79)90505-7. PMID 84687.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.