 | |
| Clinical data | |
|---|---|
| Trade names | Cresemba |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous (IV) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
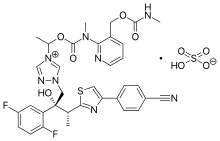
| Formula | C35H35F2N8O5S |
| Molar mass | 717.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cresemba |
| Other names | BAL8557 |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous (IV) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
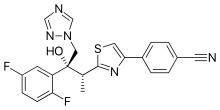
| Formula | C22H17F2N5OS |
| Molar mass | 437.47 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 122[10] °C (252 °F) |
| Solubility in water | 14.2 ± 0.5 × 10−6 mol/L (pH 7.4)[10] |
| |
| |
| | |
Isavuconazonium sulfate, sold under the brand name Cresemba, is a systemic antifungal medication of the triazole class which is used to treat invasive aspergillosis and mucormycosis.[8][6][7][11]
The most common side effects include abnormal liver tests, nausea, vomiting, difficulty breathing, abdominal pain, diarrhea, injection site reactions, headache, low blood potassium and skin rash.[9]
Isavuconazonium is a prodrug of isavuconazole.[12]
Medical uses
Isavuconazonium is used to treat invasive aspergillosis and invasive mucormycosis in adults aged eighteen years old and older. It is available in a capsule for administration by mouth and as a powder for administration via infusion.[8][6][7][11][13]
Contraindications
Isavuconazonium is contraindicated in people taking strong CYP3A4 inhibitors, strong CYP3A4 inducers, or moderate CYP3A4 or CYP3A5 inducers.[6][7][8] It is contraindicated in people with familial short QT syndrome.[6][7][8]
Side effects
Common adverse effects (occurring in between 1 and 10% of people) include low potassium, decreased appetite, delirium, headache, sleepiness, vein inflammation, difficulty breathing, acute respiratory failure, vomiting, diarrhea, nausea, stomach pain, elevated results in liver function tests, rash, itchy skin, kidney failure, chest pain, and fatigue. There are several uncommon side effects as well.[6][7]
In preclinical studies, isavuconazonium caused birth defects in animals; it has not been tested in pregnant women.[6][7][8]
Interactions
Isavuconazonium is converted into isavuconazole inside the body, and isavuconazole is a substrate for CYP3A4 or CYP3A5. Many other medications inhibit or induce those two enzymes, and isavuconazonium should not be administered with them. Inducers result in levels of isavuconazole that are too low and won't work, and inhibitors can cause high levels of isavuconazole which will in turn cause increased adverse events and toxicity. Likewise isavuconazonium can interfere with appropriate dosing of other drugs that are substrates for those enzymes.[6][7]
In addition, isavuconazole induces CYP2B6 and can decrease the amount of drugs that are metabolized by the enzyme. Isavuconazole inhibits P-glycoprotein (P-gp), BCRP, SLC22A2, and uridine diphosphate-glucuronosyltransferases, each of which remove drugs from circulation; isavuconazonium will increase the amount of drugs that are affected by those proteins and may increase their toxicities.[6][7]
Pharmacology
After oral or intravenous (IV) administration, isavuconazonium is rapidly hydrolysed by esterases in blood or the gastrointestinal tract to the active form, isavuconazole.[6][7][14]
Isavuconazole works by inhibition of lanosterol 14α-demethylase, the enzyme responsible for converting lanosterol to ergosterol by demethylation. The resulting depletion of ergosterol and buildup of lanosterol compromise the structure of the fungal cell membrane. Mammalian cells are resistant to demethylation inhibition by azoles, making the drug effects specific to fungi.[6][7][14]
Chemistry
Isavuconazonium comprises an N-(3-acetoxypropyl)-N-methylamino-carboxymethyl group linked through an ester moiety to the triazole nitrogen in isavuconazole.[15][16][17] In the aquatic media of the body, the isavuconazole molecule is transformed into monohydrate.[10]
History
Isavuconazole and isavuconazonium were discovered in Japan by researchers at Roche's research center in Kamakura.[15][18] Basilea Pharmaceutica, which had been spun out of Roche to develop antimicrobial assets, developed isavuconazonium through Phase II clinical trials. In February 2010, Basilea partnered with Astellas Pharma to complete Phase III trials, obtain regulatory approvals, and market the drug. In 2013 and 2014, the partners won orphan drug designation in the US for isavuconazonium for treating invasive aspergillosis, mucormycosis, and invasive candidiasis.[14][19][20][21]
In 2014, Basilea and Astellas amended the agreement to give Astellas sole marketing authority in North America, and Basilea the rights to market in the rest of the world.[22]
The U.S. Food and Drug Administration (FDA) granted approval in March 2015,[14][23] and the European Medicines Agency (EMA) approved it in October 2015.[9][6][7]
In 2017, Basilea licensed rights to Pfizer to market isavuconazole in Europe and other regions.[24][25][26]
References
- 1 2 "Isavuconazonium (Cresemba) Use During Pregnancy". Drugs.com. 31 January 2020. Retrieved 26 August 2020.
- ↑ "Cresemba isavuconazole (as isavuconazonium sulfate) 200 mg powder for injection vial". Therapeutic Goods Administration (TGA). ARTG ID 305480. Archived from the original on 19 June 2022. Retrieved 5 September 2021.
- ↑ "Cresemba isavuconazole (as isavuconazonium sulfate) 100 mg capsule blister pack". Therapeutic Goods Administration (TGA). ARTG ID 305452. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- ↑ "AusPAR: Isavuconazole (as sulphate)". Therapeutic Goods Administration (TGA). 22 January 2020. Retrieved 5 September 2021.
- ↑ "Search Page - Drug and Health Product Register". 23 October 2014.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Cresemba 100 mg hard capsules - Summary of Product Characteristics (SmPC)". (emc). 28 June 2021. Retrieved 5 September 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Cresemba 200mg Powder for concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). 28 June 2021. Retrieved 5 September 2021.
- 1 2 3 4 5 6 "Cresemba- isavuconazonium sulfate capsule Cresemba- isavuconazonium sulfate injection, powder, lyophilized, for solution". DailyMed. 2 December 2019. Retrieved 26 August 2020.
- 1 2 3 "Cresemba EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 26 August 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 Voronin AP, Vasilev NA, Surov AO, Churakov AV, Perlovich GL (2021). "Exploring the solid form landscape of the antifungal drug isavuconazole: crystal structure analysis, phase transformation behavior and dissolution performance". CrystEngComm. 23 (48): 8513–8526. doi:10.1039/D1CE01353J. S2CID 244426797.
- 1 2 Donnelley MA, Zhu ES, Thompson GR (2 June 2016). "Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections". Infection and Drug Resistance. 9: 79–86. doi:10.2147/IDR.S81416. PMC 4898026. PMID 27330318.
- ↑ Wilkes GM, Barton-Burke M (2019). 2020-2021 Oncology Nursing Drug Handbook. Jones & Bartlett Learning. pp. 1874–1876. ISBN 978-1-284-17132-7.
- ↑ Miceli MH, Kauffman CA (November 2015). "Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent". Clinical Infectious Diseases. 61 (10): 1558–65. doi:10.1093/cid/civ571. PMID 26179012.
- 1 2 3 4 Pettit NN, Carver PL (July 2015). "Isavuconazole: A New Option for the Management of Invasive Fungal Infections". The Annals of Pharmacotherapy. 49 (7): 825–42. doi:10.1177/1060028015581679. PMID 25940222. S2CID 208875031.
- 1 2 Guinea J, Bouza E (December 2008). "Isavuconazole: a new and promising antifungal triazole for the treatment of invasive fungal infections". Future Microbiology. 3 (6): 603–15. doi:10.2217/17460913.3.6.603. PMID 19072177.
- ↑ "Proposed INN: List 96" (PDF). WHO Drug Information. 20 (4). 2006.
- ↑ "Recommended INN: List 58" (PDF). WHO Drug Information. 21 (3). 2007.
- ↑ Ohwada J, Tsukazaki M, Hayase T, Oikawa N, Isshiki Y, Fukuda H, et al. (January 2003). "Design, synthesis and antifungal activity of a novel water soluble prodrug of antifungal triazole". Bioorganic & Medicinal Chemistry Letters. 13 (2): 191–6. doi:10.1016/s0960-894x(02)00892-2. PMID 12482421.
- ↑ "Isavuconazonium sulfate Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 6 May 2013. Retrieved 26 August 2020.
- ↑ "Isavuconazonium sulfate Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 20 October 2014. Retrieved 26 August 2020.
- ↑ "Isavuconazonium sulfate Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 25 October 2013. Retrieved 26 August 2020.
- ↑ "Astellas Takes Over Isavuconazole Manufacturing and Promotion in U.S., Canada". Genetic Engineering & Biotechnology News. 28 February 2014.
- ↑ "Cresemba Capsules & Cresemba Powder for Injection". U.S. Food and Drug Administration (FDA). 6 March 2015. Retrieved 26 August 2020.
- Lay summary in: "207500Orig1s000 / 207501Orig1s000 Labeling" (PDF). Center for Drug Evaluation and Research. 2015.
- ↑ Elvidge S (16 June 2017). "Pfizer builds out anti-infective reach with Basilea deal". BioPharma Dive.
- ↑ "Pfizer Receives Exclusive Commercialization Rights in Europe for Cresemba, a Novel Treatment for Potentially Life-Threatening Fungal Infections Among Immunocompromised Patients". Pfizer (Press release). 14 June 2017. Retrieved 5 September 2021.
- ↑ "Pfizer Enters into Agreement to Develop and Commercialize Cresemba (isavuconazole) in China and Asia Pacific Region". Pfizer (Press release). 30 November 2017. Retrieved 5 September 2021.
External links
- "Isavuconazonium". Drug Information Portal. U.S. National Library of Medicine.
- "Isavuconazonium sulfate". Drug Information Portal. U.S. National Library of Medicine.
- "Isavuconazole". Drug Information Portal. U.S. National Library of Medicine.