 | |
| Clinical data | |
|---|---|
| Trade names | Viadril, Predion, Presuren |
| Other names | 21-Hydroxy-5β-pregnane-3,20-dione |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
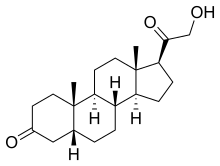
| Formula | C21H32O3 |
| Molar mass | 332.484 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Hydroxydione, as hydroxydione sodium succinate (INN, USAN, BAN) (brand names Viadril, Predion, and Presuren),[2][3][4] also known as 21-Hydroxy-5β-pregnane-3,20-dione, is a neuroactive steroid which was formerly used as a general anesthetic, but was discontinued due to incidence of thrombophlebitis in patients.[5] It was introduced in 1957,[4] and was the first neuroactive steroid general anesthetic to be introduced for clinical use, an event which was shortly preceded by the observation in 1954 of the sedative properties of progesterone in mice.[6]
Chemistry
Related compounds include alfadolone, alfaxolone, dihydrodeoxycorticosterone, ganaxolone, minaxolone, pregnanolone, and renanolone.
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 531–. ISBN 978-3-88763-075-1.
- ↑ Kar A (1 January 2005). Medicinal Chemistry. New Age International. pp. 63–. ISBN 978-81-224-1565-0.
- 1 2 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1863–. ISBN 978-0-8155-1856-3.
- ↑ Edmond II IE, Saidman L, Westhorpe R (14 September 2013). The Wondrous Story of Anesthesia. Springer Science & Business Media. pp. 632–. ISBN 978-1-4614-8441-7.
- ↑ Dorfman RI (22 October 2013). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 447–. ISBN 978-1-4832-7299-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.