 | |
| Names | |
|---|---|
| IUPAC name
1H-benzo[g]pteridine-2,4-dione | |
| Other names
1,2,3,4-Tetrahydrobenzopteridine-2,4-dione; Benzo(g)pteridine-2,4(1H,3H)-dione | |
| Identifiers | |
3D model (JSmol) |
|
| 85819 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.014 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H6N4O2 | |
| Molar mass | 214.184 g·mol−1 |
| Appearance | Red solid |
| Melting point | 200 °C (392 °F; 473 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
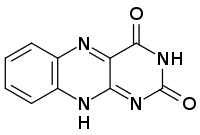
Isoalloxazine is the structural foundation of flavins such as riboflavin (vitamin B2) and is a heterocyclic compound.[2] It has a tricyclic structure which means it has three interconnected rings of atoms and is a tautomer of alloxazine.[1] The structure is formed by primary-secondary aromatic o-diamines and they are a high-melting crystalline substance.[1] The R-group is used to attach various flavin groups It has a similar structure to pteridines which has two interconnected rings.[1] Isoalloxazine was first obtained in 1934[1] by Richard Kuhn an Austrian-German biochemist and lab mates.

Isoalloxazine ring
Isoalloxazine rings can exist in different redox and ionization states depending on the chemistry of FMN and FAD associated with it.[3] Using the redox-active isoalloxazine system, FAD and FMN are able to do one and two electron transfer reactions and also be coupled with proton transfers [4]

References
- 1 2 3 4 5 Berezovskii, VM; Eremenko, TV (1963). "Chemistry of Alloxazines and Isoalloxazines". Russian Chemical Reviews. 32 (6): 290–307. doi:10.1070/RC1963v032n06ABEH001343. Retrieved November 23, 2022.
- ↑ "isoalloxazine". Farlex Partner Medical Dictionary. 2012. Retrieved November 25, 2022.
- ↑ Luliano, James N. (2019). "Vibrational spectroscopy of flavoproteins". New Approaches for Flavin Catalysis. Methods in Enzymology. Vol. 620 (volume 620 ed.). Methods in Enzymology: Elsevier Inc. pp. 189–214. doi:10.1016/bs.mie.2019.03.011. ISBN 9780128168295. ISSN 0076-6879. PMID 31072487. S2CID 146800749.
- ↑ Aleksandrov, Alexey (2019). "A Molecular Mechanics Model for Flavins". Journal of Computational Chemistry. 40 (32): 2834–2842. doi:10.1002/jcc.26061. PMID 31471978. S2CID 201730443.