 | |
| Names | |
|---|---|
| IUPAC name
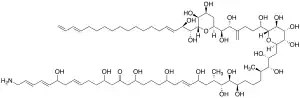
(2E,4E,8E,22E,26S,27R,28S,29R,33R,34R,35R)-1-amino-36-((2R,3S,4R,5S,6R)-6-((5R,6R)-6-((2S,4S,5S,6S)-6-((1R,2R,3E,15E)-1,2-dihydroxyoctadeca-3,15,17-trien-1-yl)-4,5-dihydroxytetrahydro-2H-pyran-2-yl)-1,5,6-trihydroxy-4-methylenehexyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)-6,12,16,20,24,27,28,29,34,35-decahydroxy-26,33-dimethylhexatriaconta-2,4,8,22-tetraen-14-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C73H127ClNO23 | |
| Molar mass | 1422.25 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Karmitoxin is an amine-containing polyhydroxy-polyene toxin isolated from Karlodinium armiger strain K-0668.[1] It is structurally related to amphidinols, luteophanols, lingshuiols, carteraols, and karlotoxins.
See also
References
- ↑ Rasmussen, Silas Anselm; Binzer, Sofie Bjørnholt; Hoeck, Casper; Meier, Sebastian; de Medeiros, Livia Soman; Andersen, Nikolaj Gedsted; Place, Allen; Nielsen, Kristian Fog; Hansen, Per Juel; Larsen, Thomas Ostenfeld (2017-04-05). "Karmitoxin: An Amine-Containing Polyhydroxy-Polyene Toxin from the Marine Dinoflagellate Karlodinium armiger". Journal of Natural Products. American Chemical Society (ACS). 80 (5): 1287–1293. doi:10.1021/acs.jnatprod.6b00860. ISSN 0163-3864. PMC 6446557. PMID 28379705.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.