 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lithium hydrosulfide | |
| Preferred IUPAC name
Lithium sulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.032.013 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Li2S | |
| Molar mass | 45.95 g/mol |
| Appearance | white solid |
| Density | 1.67 g/cm3 |
| Melting point | 938 °C (1,720 °F; 1,211 K) |
| Boiling point | 1,372 °C (2,502 °F; 1,645 K) |
| very soluble, hydrolyses to LiOH and H2S | |
| Solubility | very soluble in ethanol |
| Structure | |
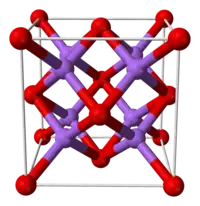
| Antifluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
| Tetrahedral (Li+); cubic (S2−) | |
| Thermochemistry | |
Std molar entropy (S⦵298) |
63 J/mol K |
Std enthalpy of formation (ΔfH⦵298) |
-9.401 kJ/g or -447 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
240 mg/kg (oral, rat)[1] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions |
Lithium oxide Lithium selenide Lithium telluride Lithium polonide |
Other cations |
Sodium sulfide Potassium sulfide Rubidium sulfide Caesium sulfide |
Related compounds |
Lithium hydrosulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
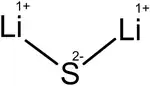
Lithium sulfide is the inorganic compound with the formula Li2S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms a solid yellow-white deliquescent powder. In air, it easily hydrolyses to release hydrogen sulfide (rotten egg odor).[2]
Preparation
Lithium sulfide is prepared by treating lithium with sulfur. This reaction is conveniently conducted in anhydrous ammonia.[3]
- 2 Li + S → Li2S
The THF-soluble triethylborane adduct of lithium sulfide can be generated using superhydride.[4]
Reactions and applications
Lithium sulfide has been considered for use in lithium–sulfur batteries.[5]
References
- ↑ https://chem.nlm.nih.gov/chemidplus/rn/12136-58-2
- ↑ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ Rankin, D. W. H. (1974). "Digermanyl Sulfide". Inorg. Synth. 15: 182–84. doi:10.1002/9780470132463.ch40. ISBN 978-0-470-13246-3.
- ↑ Gladysz, J. A.; Wong, V. K.; Jick, B. G. (1979). "New Methodology for the Introduction of Sulfur into Organic Molecules. Synthesis of Anhydrous Dilithium Dulfide, Dilithium Disulfide and Lithium Thiolates by Lithium Triethylborohydride Reduction of Elemental Sulfur and Disulfides". Tetrahedron. 35: 2329–2335. doi:10.1016/S0040-4020(01)93746-9.
- ↑ "Battery claims greater capacity than lithium ion". Electronics Weekly. 12 July 2005. Retrieved 2005-09-16.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
