 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.379 |
| Chemical and physical data | |
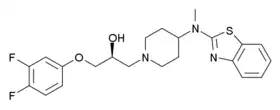
| Formula | C22H25F2N3O2S |
| Molar mass | 433.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lubeluzole (Prosynap) is a drug which acts as an indirect NMDA antagonist. It inhibits the release of glutamate, inhibits nitric oxide synthesis, and blocks calcium and sodium gated ion channels.[1] It has neuroprotective effects particularly in hypoxic conditions,[2][3] and was developed for the treatment of stroke.[4] Trials showed it to be safe, effective and well tolerated at low doses, but unfortunately higher doses produced the dangerous cardiac side effect of lengthening the QTc interval,[5][6] which could potentially lead to heart failure, and so this meant that subsequent trials were limited to using only the low dose range.[7] Animal studies had shown lubeluzole to produce neuroprotective effects when administered for prolonged periods, but the aim of its developers was to produce a drug that would be effective for preventing damage from acute stroke, and so ultimately it failed to show sufficient efficacy in trials and development for medical use was halted.[8]
References
- ↑ Culmsee C, Junker V, Wolz P, Semkova I, Krieglstein J (January 1998). "Lubeluzole protects hippocampal neurons from excitotoxicity in vitro and reduces brain damage caused by ischemia". European Journal of Pharmacology. 342 (2–3): 193–201. doi:10.1016/s0014-2999(97)01499-4. PMID 9548385.
- ↑ Ashton D, Willems R, Wynants J, Van Reempts J, Marrannes R, Clincke G (January 1997). "Altered Na(+)-channel function as an in vitro model of the ischemic penumbra: action of lubeluzole and other neuroprotective drugs". Brain Research. 745 (1–2): 210–21. doi:10.1016/s0006-8993(96)01094-3. PMID 9037412. S2CID 27971291.
- ↑ Maiese K, TenBroeke M, Kue I (February 1997). "Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide". Journal of Neurochemistry. 68 (2): 710–4. doi:10.1046/j.1471-4159.1997.68020710.x. PMID 9003060. S2CID 15532366.
- ↑ Grotta J (December 1997). "Lubeluzole treatment of acute ischemic stroke. The US and Canadian Lubeluzole Ischemic Stroke Study Group". Stroke. 28 (12): 2338–46. doi:10.1161/01.str.28.12.2338. PMID 9412611.
- ↑ Hantson L, Tritsmans L, Crabbé R, Gheuens J, Van Rooy P (November 1997). "The safety and tolerability of single intravenous doses of lubeluzole (Prosynap) in healthy volunteers". International Journal of Clinical Pharmacology and Therapeutics. 35 (11): 491–5. PMID 9401829.
- ↑ Wood PL, Hawkinson JE (April 1997). "N-methyl-D-aspartate antagonists for stroke and head trauma". Expert Opinion on Investigational Drugs. 6 (4): 389–97. doi:10.1517/13543784.6.4.389. PMID 15989606.
- ↑ Hacke W, Lees KR, Timmerhuis T, Haan J, Hantson L, Hennerici M, Diener HC (1998). "Cardiovascular safety of lubeluzole (Prosynap(R)) in patients with ischemic stroke". Cerebrovascular Diseases. 8 (5): 247–54. doi:10.1159/000015861. PMID 9712921. S2CID 25262626.
- ↑ Gandolfo C, Sandercock P, Conti M (2002). "Lubeluzole for acute ischaemic stroke". The Cochrane Database of Systematic Reviews (1): CD001924. doi:10.1002/14651858.CD001924. PMID 11869612.