 | |
| Names | |
|---|---|
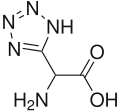
| IUPAC name
(RS)-Amino(1H-tetrazol-5-yl)acetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H5N5O2 | |
| Molar mass | 143.106 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tetrazolylglycine (Tet-Gly, LY-285,265) is a potent and selective NMDA receptor agonist, stimulating the NMDA receptor with higher potency than either glutamate or NMDA.[1] It is a potent convulsant and excitotoxin and is used in scientific research.[2][3]
References
- ↑ Lunn WH, Schoepp DD, Calligaro DO, Vasileff RT, Heinz LJ, Salhoff CR, O'Malley PJ (1992). "DL-tetrazol-5-ylglycine, a highly potent NMDA agonist: its synthesis and NMDA receptor efficacy". Journal of Medicinal Chemistry. 35 (24): 4608–12. doi:10.1021/jm00102a015. PMID 1361579.
- ↑ Schoepp DD, Smith CL, Lodge D, Millar JD, Leander JD, Sacaan AI, Lunn WH (1991). "D,L-(tetrazol-5-yl) glycine: a novel and highly potent NMDA receptor agonist". European Journal of Pharmacology. 203 (2): 237–43. doi:10.1016/0014-2999(91)90719-7. PMID 1686860.
- ↑ Schoepp DD, Lunn WH, Salhoff CR, McDonald JW (1994). "The NMDA receptor agonist DL-(tetrazol-5-yl)glycine is a highly potent excitotoxin". European Journal of Pharmacology. 270 (1): 67–72. doi:10.1016/0926-6917(94)90081-7. PMID 8157082.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.