 | |
| Names | |
|---|---|
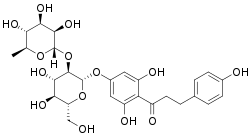
| IUPAC name
1-[4-[(3S,4R,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-2, 6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one | |
| Systematic IUPAC name
3,5-Dihydroxy-4-[3-(4-hydroxyphenyl)propanoyl]phenyl 2-O-(6-deoxy-α-L-mannopyranosyl)-β-L-glucopyranoside | |
| Other names
Naringin DC | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.127.977 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C27H34O14 | |
| Molar mass | 582.555 g·mol−1 |
| Appearance | White powder |
| Melting point | 169 to 170 °C (336 to 338 °F; 442 to 443 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Naringin dihydrochalcone, sometimes abbreviated to naringin DC, is an artificial sweetener derived from naringin, a bitter compound found in citrus.[1]
Naringin dihydrochalcone is a phloretin glycoside discovered at the same time as neohesperidin dihydrochalcone during the 1960s as part of a United States Department of Agriculture research program to find methods for minimizing the taste of bitter flavorants in citrus juices.
When naringin is treated with potassium hydroxide or another strong base, and then catalytically hydrogenated, it becomes a dihydrochalcone that is roughly 300–1800 times sweeter than sugar at threshold concentrations.[2]
References
- ↑ Ikan, R. (1991). "1-Flavonoides. E. Synthesis of Naringin Dihydrochalcone — A Sweetening Agent". Natural Products: A Laboratory Guide (2nd ed.). Academic Press. pp. 17–18. ISBN 0-12-370551-7.
- ↑ Tomasik, P., ed. (2003). Chemical and Functional Properties of Food Saccharides. Boca Raton: CRC Press. p. 389. ISBN 978-0-84-931486-5.
External links
 Media related to Naringin dihydrochalcone at Wikimedia Commons
Media related to Naringin dihydrochalcone at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.