Receptor-type tyrosine-protein phosphatase kappa is an enzyme that in humans is encoded by the PTPRK gene.[5][6][7] PTPRK is also known as PTPkappa and PTPκ.
Function
The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. Protein tyrosine phosphatases are protein enzymes that remove phosphate moieties from tyrosine residues on other proteins. Tyrosine kinases are enzymes that add phosphates to tyrosine residues, and are the opposing enzymes to PTPs. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation.
The human PTPRK gene is located on the long arm of chromosome 6, a putative tumor suppressor region of the genome.[8]
During development
The same reporter construct used by Shen and colleagues, and described above was created by Skarnes et al. during a screen to identify genes important in mouse development.[9] The transgenic mouse was created by combining a β-galactosidase (β-gal) reporter gene with a signal sequence and the transmembrane domain of the type I transmembrane protein CD4. If the transgene was incorporated into a gene with a signal sequence, β-gal activity would remain in the cytosol of the cell and therefore be active. If the reporter gene was incorporated into a gene that lacked a signal sequence, β-gal activity would be in the ER where it would lose β-gal activity. This construct inserted into the phosphatase domain of PTPkappa.[10] Mice generated from these ES cells were viable, suggesting that PTPkappa phosphatase activity is not necessary for embryonic development.[9][10]
Additional studies have suggested a function for PTPkappa during nervous system development. PTPkappa promotes neurite outgrowth from embryonic cerebellar neurons, and thus may be involved in axonal extension or guidance in vivo.[11] Neurites are extensions from neurons that can be considered the in vitro equivalent of axons and dendrites. The extension of cerebellar neurites on purified PTPkappa fusion proteins was demonstrated to require Grb2 and MEK1 activity.[11]
In T cells
PTPkappa has also been shown to regulate CD4+ positive T cell development.[12] PTPkappa and the THEMIS gene are both deleted in the rat Long-Evans Cinnamon (LEC) strain, and are both required for the CD4+ T-cell deficiency observed in this strain of rats.[12][13] Deletion of PTPkappa was shown to generate T-helper immunodeficiency in the LEC strain.[14]
By expressing a dominant negative form of PTPkappa or by using short-hairpin RNA for PTPkappa in bone-marrow derived stem cells, CD4(+) T cells development is inhibited.[15] PTPkappa likely regulates T-cell development by positively regulating ERK1/2 phosphorylation via the regulation of MEK1/2 and c-Raf phosphorylation.[15]
Cadherin-catenin signaling
PTPkappa is localized to cell-cell contact sites, where it colocalizes and co-immunoprecipitates with β-catenin and plakoglobin/γ-catenin[6] β-catenin may be a PTPkappa substrate.[6][16] The presence of full-length PTPkappa in melanoma cells decreases the level of free-cytosolic β-catenin, which consequently reduces the level of nuclear β-catenin and reduces the expression of the β-catenin-regulated genes, cyclin D1 and c-myc.[17] Expression of ful-length PTPkappa in melanoma cells that normally lack its expression results in reduced cell migration and cell proliferation. Because the presence of PTPkappa at the cell membrane was shown to sequester β-catenin to the plasma membrane, these data suggest that one mechanism whereby PTPkappa functions as a tumor suppressor is by regulating the intracellular localization of free-β-catenin.[17]
The intracellular fragments of PTPkappa, PΔE and PIC, are catalytically active, and can also dephosphorylate β-catenin.[16] Tyrosine phosphorylated β-catenin translocates to the cell nucleus and activates TCF-mediated transcription to promote cell proliferation and migration. While full-length PTPkappa antagonizes TCF-mediated transcription, the PIC fragment augments it, perhaps by regulating other proteins in TCF-mediated transcription.[16] This suggests that phosphatase activity of the PIC fragment opposes that of full-length PTPkappa.[16]
PTPkappa interacts by co-immunoprecipitation with E-cadherin, α-catenin and β-catenin in pancreatic acinar cells prior to the dissolution of adherens junctions in a rat model of pancreatitis.[18] The authors suggest that the presence of PTPkappa at the plasma membrane in association with the cadherin/catenin complex is important for the maintenance of adherens junction in pancreatic acinar cells, much as it was suggested above in melanoma cells.[18]
EGFR signaling
Use of short interfering RNA (siRNA) of PTPkappa to reduce PTPkappa protein expression in the mammary epithelial cell line, MCF10A, resulted in increased cell proliferation.[19] PTPkappa expression, conversely, was demonstrated to reduce cell proliferation in Chinese hamster ovary cells.[20] The mechanism proposed to explain the influence of PTPkappa on cell proliferation is via PTPkappa dephosphorylation of the EGFR on tyrosines 1068 and 1173 directly. The reduction of PTPkappa expression in CHO cells with PTPkappa siRNA increased EGFR phosphorylation.[20] Therefore, the hypothesis is that PTPkappa functions as a tumor suppressor gene by dephosphorylating and inactivating EGFR.[20]
In addition, glycosylation by N-acetylglucosaminyltransferase-V (GnT-V) has been shown to reduce full-length PTPkappa expression, likely via increasing its cleavage.[21] This aberrant glycosylation has been shown to increase the phosphorylation of EGFR on tyrosine 1068, likely because of reduced plasma-membrane associated PTPkappa expression and hence reduced PTPkappa-mediated dephosphorylation of its membrane associated substrates, such as EGFR.[22]
Structure
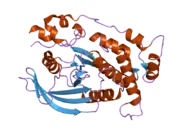
PTPkappa possesses an extracellular region, a single transmembrane region, and two tandem catalytic domains, and thus represents a receptor-type PTP (RPTP). The extracellular region contains a meprin-A5 antigen-PTP mu (MAM) domain, an Ig-like domain and four fibronectin type III-like repeats.[23] PTPkappa is a member of the R2B subfamily of RPTPs, which includes RPTPM, RPTPT, and RPTPU. PTPkappa shares most sequence similarity with PTPmu and PTPrho.
Crystal structure analysis of the first phosphatase domain of PTPkappa demonstrates that it shares many conformational features with PTPmu, including an unhindered open conformation for the catalytically important WPD loop, and a phosphate binding loop for the active-site cysteine (Cys1083). PTPkappa exists as a monomer in solution, with the caveat that dimers of PTPkappa are observed depending on the nature of the buffer used.[24]
Alternative splicing
Alternative splicing of exons 16, 17a, and 20a has been described for PTPRK.[25] Two novel forms of PTPRK were identified from mouse full-length cDNA sequences and were predicted to result in two PTPkappa splice variants: a secreted form of PTPkappa and a membrane tethered form.[26]
Homophilic binding
PTPkappa mediates homophilic cell-cell aggregation via its extracellular domain.[27] PTPkappa only mediates binding between cells expressing PTPkappa (i.e. homophilic), and will not mediate cell aggregation between cells expressing PTPkappa, PTPmu or PTPrho (i.e. heterophilic).[28][29]
Regulation
Proteolysis and N-glycosylation
Full-length PTPkappa protein is cleaved by furin to generate two cleaved fragments that remain associated at the plasma membrane, an extracellular (E) subunit and an intracellular phosphatase (P) subunit.[6][23] In response to high cell density or calcium influx following trifluoperazine (TFP) stimulation, PTPkappa is further cleaved by ADAM 10 to yield a shed extracellular fragment and a membrane tethered intracellular fragment, PΔE.[16] The membrane tethered PΔE fragment is further cleaved by the gamma secretase complex to yield a membrane-released fragment, PIC, that can translocate to the cellular nucleus, where it is catalytically active.[16]
Glycosylation of the extracellular domain of PTPkappa was demonstrated to occur preferentially in WiDr colon cancer cells that over-express N-acetylglucosaminyl transferase V (GnT-V).[21] Over-expression of GnT-V in these cells increased the cleavage and shedding of PTPkappa ectodomain and increased migration of WiDr cells in transwell assays.[21] As a result of glycosylation of PTPkappa by GnT-V, EGFR was phosphorylated on tyrosine 1068 and activated, and is likely the cause of the increased cell migration observed following PTPkappa cleavage.[22]
Shedding of PTPkappa may also be regulated by the presence of galectin-3 binding protein, as has been shown in WiDr cells.[30] The authors suggest that the ratio of galectin-3 binding protein to galectin 3 influences the cleavage and shedding of PTPkappa, although the exact mechanism of how these proteins regulate PTPkappa cleavage was not determined.
By reactive oxygen species in cancer
One mechanism whereby PTPkappa tyrosine phosphatase activity can be perturbed in cancer is via oxidative inhibition mediated by reactive oxygen species generated by either hydrogen peroxide in vitro or UV irradiation of skin cells in vivo.[31] In cell-free assays, the presence of hydrogen peroxide reduces PTPkappa tyrosine phosphatase activity and increases EGFR tyrosine phosphorylation.[31] UV-irradiation of primary human keratinocytes yields the same results, namely a reduction of PTPkappa tyrosine phosphatase activity and an increase in EGFR tyrosine phosphorylation. EGFR phosphorylation then leads to cell proliferation, suggesting that PTPkappa may function as a tumor suppressor in skin cancer in addition to melanoma.[31]
Expression
PTPkappa is expressed in human keratinocytes. TGFβ1 is a growth inhibitor in human keratinocytes. Stimulation of the cultured human keratinocyte cell line, HaCaT, with TGFβ1 increases the levels of PTPkappa (PTPRK) mRNA as assayed by northern blot analysis.[32] TGFβ1 also increased PTPkappa mRNA and protein in normal and tumor mammary cell lines.[19] HER2 overexpression reduced PTPkappa mRNA and protein expression.[19]
Clinical significance
Melanoma and skin cancer
Expression analysis of PTPkappa mRNA in normal melanocytes and in melanoma cells and tissues demonstrated that PTPkappa is downregulated or absent 20% of the time in melanoma, suggesting that PTPkappa is a tumor suppressor gene in melanoma.[33] A form of PTPkappa with a point mutation in the fourth fibronectin III repeat was identified to be a melanoma specific antigen recognized by CD4+ T cells in a melanoma patient with 10-year tumor-free survival after lymph node resection.[34] This particular mutated form of PTPkappa was not identified in 10 other melanoma cell lines, and may thus represent a unique mutation in one patient.[34]
Lymphoma
PTPkappa was also identified as the putative tumor suppressor gene commonly deleted in primary central nervous system lymphomas (PCNSLs).[35]
Downregulation of PTPkappa was found to occur following Epstein-Barr Virus (EBV) infection of Hodgkin's Lymphomas cells.[36]
Colorectal cancer
Using a transposon-based genetic screen, researchers found that disruption of the PTPRK gene in gastrointestinal tract epithelium resulted in an intestinal lesion, classified as either an intraepithelial neoplasia, an adenocarcinoma or an adenoma.[37]
Lung cancer
PTPRK mRNA was shown to be significantly reduced by RT-PCR in human lung cancer-derived cell lines.[38]
Prostate cancer
PTPRK has also been shown to be downregulated in response to androgen stimulation in human LNCaP prostate cancer cells.[39] The mechanism whereby PTPRK is downregulated is via the expression of a microRNA, miR-133b, which is upregulated in response to androgen stimulation.[39]
Breast cancer
Patients with reduced PTPRK transcript expression have shorter breast cancer survival times and are more likely to have breast cancer metastases or to die from breast cancer.[40] In an experimental model of breast cancer, PTPRK was reduced in breast cancer cell lines with PTPRK ribozymes.[40] In these cells, adhesion to matrigel, transwell migration, and cell growth were all increased following the reduction of PTPRK expression, again supporting a function for PTPRK as a tumor suppressor.[40]
Glioma
Assem and colleagues identified loss of heterozygosity (LOH) events in malignant glioma specimens, and identified PTPRK as a significant gene candidate in one LOH region.[41] A significant correlation between the presence of PTPRK mutations and short patient survival time was observed.[41] PTPRK was amplified from tumor cDNA to confirm the LOH observed. In these specimens, 6 different mutations were observed, two of which (one in each phosphatase domain) disrupted the enzymatic activity of PTPRK.[42] Expression of wild-type PTPkappa in U87-MG and U251-MG cells resulted in a reduction in cell proliferation, migration and invasion.[42] Expression of the variants of PTPkappa with mutations in the phosphatase domains, however, increased cell proliferation, migration and invasion, supporting a role for the involvement of the mutated variants of PTPkappa in tumorigenicity.[42]
In development
In situ hybridization localized PTPkappa mRNA to the brain, lung, skeletal muscle, heart, placenta, liver, kidney and intestines during development.[43] PTPkappa was also found to be expressed in the developing retina, in nestin-positive radial progenitor cells and later in development, in the ganglion cell layer, inner plexiform layer and outer segments of photoreceptors.[44] PTPkappa protein is observed in neural progenitor cells and radial glial cells of the developing mouse superior colliculus, as well.[45]
In the adult rat brain, PTPkappa mRNA is highly expressed in regions of the brain with cellular plasticity and growth, such as the olfactory bulb, the hippocampus and the cerebral cortex.[23] PTPkappa mRNA is also observed in the adult mouse cerebellum.[25]
Using a β-galactosidase (β-gal) reporter gene inserted into the phosphatase domain of the murine PTPkappa (PTPRK) gene, Shen and colleagues determined the detailed expression pattern of endogenous PTPRK.[10] β-gal activity was observed in many areas of the adult forebrain, including layers II and IV, and to a lesser extent in layer VI of the cortex. β-gal activity was also observed in apical dendrites of cortical pyramidal cells, the granule layer of the olfactory and accessory olfactory bulbs, the anterior hypothalamus, paraventricular nucleus, and in granule and pyramidal layers of the dentate gyrus and CA 1-3 regions of the hippocampus.[10] In the midbrain, β-gal was observed in the subthalamic nucleus, the superior and inferior colliculi and in the red nucleus. β-gal activity was also observed in the neural retina, in the inner nuclear layer and in small ganglion cells of the ganglion cell layer.[10]
Interactions
PTPRK has been shown to interact with:
- Beta-catenin,[6][16][18]
- E-cadherin (CDH-1),[18]
- Epidermal growth factor receptor (EGFR),[19]
- HER2,[19]
- Plakoglobin,[6] and
- α-catenin.[18]
References
- 1 2 3 ENSG00000273993 GRCh38: Ensembl release 89: ENSG00000152894, ENSG00000273993 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000019889 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Yang Y, Gil MC, Choi EY, Park SH, Pyun KH, Ha H (Mar 1997). "Molecular cloning and chromosomal localization of a human gene homologous to the murine R-PTP-kappa, a receptor-type protein tyrosine phosphatase". Gene. 186 (1): 77–82. doi:10.1016/S0378-1119(96)00684-1. PMID 9047348.
- 1 2 3 4 5 6 Fuchs M, Müller T, Lerch MM, Ullrich A (Aug 1996). "Association of human protein-tyrosine phosphatase kappa with members of the armadillo family". J Biol Chem. 271 (28): 16712–9. doi:10.1074/jbc.271.28.16712. PMID 8663237.
- ↑ "Entrez Gene: PTPRK protein tyrosine phosphatase, receptor type, K".
{{cite web}}: Missing or empty|url=(help) - ↑ Zhang Y, Siebert R, Matthiesen P, Yang Y, Ha H, Schlegelberger B (1998). "Cytogenetical assignment and physical mapping of the human R-PTP-kappa gene (PTPRK) to the putative tumor suppressor gene region 6q22.2-q22.3". Genomics. 51 (2): 309–11. doi:10.1006/geno.1998.5323. PMID 9722959.
- 1 2 Skarnes WC, Moss JE, Hurtley SM, Beddington RS (1995). "Capturing genes encoding membrane and secreted proteins important for mouse development". Proc Natl Acad Sci U S A. 92 (14): 6592–6. Bibcode:1995PNAS...92.6592S. doi:10.1073/pnas.92.14.6592. PMC 41564. PMID 7604039.
- 1 2 3 4 5 Shen P, Canoll PD, Sap J, Musacchio JM (1999). "Expression of a truncated receptor protein tyrosine phosphatase kappa in the brain of an adult transgenic mouse". Brain Res. 826 (2): 157–71. doi:10.1016/s0006-8993(99)01179-8. PMID 10224293. S2CID 40530391.
- 1 2 Drosopoulos NE, Walsh FS, Doherty P (1999). "A soluble version of the receptor-like protein tyrosine phosphatase kappa stimulates neurite outgrowth via a Grb2/MEK1-dependent signaling cascade". Mol Cell Neurosci. 13 (6): 441–9. doi:10.1006/mcne.1999.0758. PMID 10383829. S2CID 35458154.
- 1 2 Kose H, Sakai T, Tsukumo S, Wei K, Yamada T, Yasutomo K, Matsumoto K (2007). "Maturational arrest of thymocyte development is caused by a deletion in the receptor-like protein tyrosine phosphatase kappa gene in LEC rats". Genomics. 89 (6): 673–7. doi:10.1016/j.ygeno.2007.03.001. PMID 17434290. S2CID 28407283.
- ↑ Iwata R, Sasaki N, Agui T (2010). "Contiguous gene deletion of Ptprk and Themis causes T-helper immunodeficiency (thid) in the LEC rat". Biomed Res. 31 (1): 83–7. doi:10.2220/biomedres.31.83. PMID 20203423.
- ↑ Asano A, Tsubomatsu K, Jung CG, Sasaki N, Agui T (2007). "A deletion mutation of the protein tyrosine phosphatase kappa (Ptprk) gene is responsible for the CD4+ T-cell immunodeficiency (thid) in the LEC rat" (PDF). Mamm Genome. 18 (11): 779–86. doi:10.1007/s00335-007-9062-0. hdl:2115/33866. PMID 17909891. S2CID 20866657.
- 1 2 Erdenebayar N, Maekawa Y, Nishida J, Kitamura A, Yasutomo K (2009). "Protein-tyrosine phosphatase-kappa regulates CD4+ T cell development through ERK1/2-mediated signaling". Biochem Biophys Res Commun. 390 (3): 489–93. doi:10.1016/j.bbrc.2009.09.117. PMID 19800317.
- 1 2 3 4 5 6 7 Anders L, Mertins P, Lammich S, Murgia M, Hartmann D, Saftig P, Haass C, Ullrich A (2006). "Furin-, ADAM 10-, and gamma-secretase-mediated cleavage of a receptor tyrosine phosphatase and regulation of beta-catenin's transcriptional activity". Mol Cell Biol. 26 (10): 3917–34. doi:10.1128/MCB.26.10.3917-3934.2006. PMC 1489012. PMID 16648485.
- 1 2 Novellino L, De Filippo A, Deho P, Perrone F, Pilotti S, Parmiani G, Castelli C (2008). "PTPRK negatively regulates transcriptional activity of wild type and mutated oncogenic beta-catenin and affects membrane distribution of beta-catenin/E-cadherin complexes in cancer cells". Cell Signal. 20 (5): 872–83. doi:10.1016/j.cellsig.2007.12.024. PMID 18276111.
- 1 2 3 4 5 Schnekenburger J, Mayerle J, Krüger B, Buchwalow I, Weiss FU, Albrecht E, Samoilova VE, Domschke W, Lerch MM (2005). "Protein tyrosine phosphatase kappa and SHP-1 are involved in the regulation of cell-cell contacts at adherens junctions in the exocrine pancreas". Gut. 54 (10): 1445–55. doi:10.1136/gut.2004.063164. PMC 1774702. PMID 15987791.
- 1 2 3 4 5 Wang SE, Wu FY, Shin I, Qu S, Arteaga CL (2005). "Transforming growth factor {beta} (TGF-{beta})-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-{beta} function". Mol Cell Biol. 25 (11): 4703–15. doi:10.1128/MCB.25.11.4703-4715.2005. PMC 1140650. PMID 15899872.
- 1 2 3 Xu Y, Tan LJ, Grachtchouk V, Voorhees JJ, Fisher GJ (2005). "Receptor-type protein-tyrosine phosphatase-kappa regulates epidermal growth factor receptor function". J Biol Chem. 280 (52): 42694–700. doi:10.1074/jbc.M507722200. PMID 16263724.
- 1 2 3 Kim YS, Kang HY, Kim JY, Oh S, Kim CH, Ryu CJ, Miyoshi E, Taniguchi N, Ko JH (2006). "Identification of target proteins of N-acetylglucosaminyl transferase V in human colon cancer and implications of protein tyrosine phosphatase kappa in enhanced cancer cell migration". Proteomics. 6 (4): 1187–91. doi:10.1002/pmic.200500400. PMID 16404719. S2CID 6580919.
- 1 2 Wang C, Yang Y, Yang Z, Liu M, Li Z, Sun L, Mei C, Chen H, Chen L, Wang L, Zha X (2009). "EGF-mediated migration signaling activated by N-acetylglucosaminyltransferase-V via receptor protein tyrosine phosphatase kappa". Arch Biochem Biophys. 486 (1): 64–72. doi:10.1016/j.abb.2009.02.005. PMID 19236842.
- 1 2 3 Jiang YP, Wang H, D'Eustachio P, Musacchio JM, Schlessinger J, Sap J (1993). "Cloning and characterization of R-PTP-kappa, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region". Mol Cell Biol. 13 (5): 2942–51. doi:10.1128/MCB.13.5.2942. PMC 359687. PMID 8474452.
- ↑ Eswaran J, Debreczeni JE, Longman E, Barr AJ, Knapp S (2006). "The crystal structure of human receptor protein tyrosine phosphatase kappa phosphatase domain 1". Protein Sci. 15 (6): 1500–5. doi:10.1110/ps.062128706. PMC 2242534. PMID 16672235.
- 1 2 Besco J, Popesco MC, Davuluri RV, Frostholm A, Rotter A (2004). "Genomic structure and alternative splicing of murine R2B receptor protein tyrosine phosphatases (PTPkappa, mu, rho and PCP-2)". BMC Genomics. 5 (1): 14. doi:10.1186/1471-2164-5-14. PMC 373446. PMID 15040814.
- ↑ Forrest AR, Taylor DF, Crowe ML, Chalk AM, Waddell NJ, Kolle G, Faulkner GJ, Kodzius R, Katayama S, Wells C, Kai C, Kawai J, Carninci P, Hayashizaki Y, Grimmond SM (2006). "Genome-wide review of transcriptional complexity in mouse protein kinases and phosphatases". Genome Biol. 7 (1): R5. doi:10.1186/gb-2006-7-1-r5. PMC 1431701. PMID 16507138.
- ↑ Sap J, Jiang YP, Friedlander D, Grumet M, Schlessinger J (1994). "Receptor tyrosine phosphatase R-PTP-kappa mediates homophilic binding". Mol Cell Biol. 14 (1): 1–9. doi:10.1128/MCB.14.1.1. PMC 358350. PMID 8264577.
- ↑ Zondag GC, Koningstein GM, Jiang YP, Sap J, Moolenaar WH, Gebbink MF (1995). "Homophilic interactions mediated by receptor tyrosine phosphatases mu and kappa. A critical role for the novel extracellular MAM domain". J Biol Chem. 270 (24): 14247–50. doi:10.1074/jbc.270.24.14247. PMID 7782276.
- ↑ Becka S, Zhang P, Craig SE, Lodowski DT, Wang Z, Brady-Kalnay SM (2010). "Characterization of the adhesive properties of the type IIb subfamily receptor protein tyrosine phosphatases". Cell Commun Adhes. 17 (2): 34–47. doi:10.3109/15419061.2010.487957. PMC 3337334. PMID 20521994.
- ↑ Kim YS, Jung JA, Kim HJ, Ahn YH, Yoo JS, Oh S, Cho C, Yoo HS, Ko JH (2011). "Galectin-3 binding protein promotes cell motility in colon cancer by stimulating the shedding of protein tyrosine phosphatase kappa by proprotein convertase 5". Biochem Biophys Res Commun. 404 (1): 96–102. doi:10.1016/j.bbrc.2010.11.071. PMID 21094132.
- 1 2 3 Xu Y, Shao Y, Voorhees JJ, Fisher GJ (2006). "Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes". J Biol Chem. 281 (37): 27389–97. doi:10.1074/jbc.M602355200. PMC 3738260. PMID 16849327.
- ↑ Yang Y, Gil M, Byun SM, Choi I, Pyun KH, Ha H (1996). "Transforming growth factor-beta1 inhibits human keratinocyte proliferation by upregulation of a receptor-type tyrosine phosphatase R-PTP-kappa gene expression". Biochem Biophys Res Commun. 228 (3): 807–12. doi:10.1006/bbrc.1996.1736. PMID 8941358.
- ↑ McArdle L, Rafferty M, Maelandsmo GM, Bergin O, Farr CJ, Dervan PA, O'Loughlin S, Herlyn M, Easty DJ (2001). "Protein tyrosine phosphatase genes downregulated in melanoma". J Invest Dermatol. 117 (5): 1255–60. doi:10.1046/j.0022-202x.2001.01534.x. PMID 11710941.
- 1 2 Novellino L, Renkvist N, Rini F, Mazzocchi A, Rivoltini L, Greco A, Deho P, Squarcina P, Robbins PF, Parmiani G, Castelli C (2003). "Identification of a mutated receptor-like protein tyrosine phosphatase kappa as a novel, class II HLA-restricted melanoma antigen". J Immunol. 170 (12): 6363–70. doi:10.4049/jimmunol.170.12.6363. PMID 12794170.
- ↑ Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, Shimada K, Ishida E, Konishi N (2003). "Novel tumor suppressor loci on 6q22-23 in primary central nervous system lymphomas". Cancer Res. 63 (4): 737–41. PMID 12591717.
- ↑ Flavell JR, Baumforth KR, Wood VH, Davies GL, Wei W, Reynolds GM, Morgan S, Boyce A, Kelly GL, Young LS, Murray PG (2008). "Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus encoded EBNA1 contributes to the growth and survival of Hodgkin lymphoma cells". Blood. 111 (1): 292–301. doi:10.1182/blood-2006-11-059881. PMID 17720884.
- ↑ Starr TK, Allaei R, Silverstein KA, Staggs RA, Sarver AL, Bergemann TL, Gupta M, O'Sullivan MG, Matise I, Dupuy AJ, Collier LS, Powers S, Oberg AL, Asmann YW, Thibodeau SN, Tessarollo L, Copeland NG, Jenkins NA, Cormier RT, Largaespada DA (2009). "A transposon-based genetic screen in mice identifies genes altered in colorectal cancer". Science. 323 (5922): 1747–50. Bibcode:2009Sci...323.1747S. doi:10.1126/science.1163040. PMC 2743559. PMID 19251594.
- ↑ Scrima M, De Marco C, De Vita F, Fabiani F, Franco R, Pirozzi G, Rocco G, Malanga D, Viglietto G (2012). "The nonreceptor-type tyrosine phosphatase PTPN13 is a tumor suppressor gene in non-small cell lung cancer". Am J Pathol. 180 (3): 1202–14. doi:10.1016/j.ajpath.2011.11.038. PMID 22245727.
- 1 2 Mo W, Zhang J, Li X, Meng D, Gao Y, Yang S, Wan X, Zhou C, Guo F, Huang Y, Amente S, Avvedimento EV, Xie Y, Li Y (2013). "Identification of novel AR-targeted microRNAs mediating androgen signalling through critical pathways to regulate cell viability in prostate cancer". PLOS ONE. 8 (2): e56592. Bibcode:2013PLoSO...856592M. doi:10.1371/journal.pone.0056592. PMC 3579835. PMID 23451058.
- 1 2 3 Sun PH, Ye L, Mason MD, Jiang WG (2013). "Protein tyrosine phosphatase kappa (PTPRK) is a negative regulator of adhesion and invasion of breast cancer cells, and associates with poor prognosis of breast cancer". J Cancer Res Clin Oncol. 139 (7): 1129–39. doi:10.1007/s00432-013-1421-5. PMID 23552869. S2CID 20002233.
- 1 2 Assem M, Sibenaller Z, Agarwal S, Al-Keilani MS, Alqudah MA, Ryken TC (2012). "Enhancing diagnosis, prognosis, and therapeutic outcome prediction of gliomas using genomics". OMICS. 16 (3): 113–22. doi:10.1089/omi.2011.0031. PMC 3300066. PMID 22401657.
- 1 2 3 Agarwal S, Al-Keilani MS, Alqudah MA, Sibenaller ZA, Ryken TC, Assem M (2013). "Tumor derived mutations of protein tyrosine phosphatase receptor type k affect its function and alter sensitivity to chemotherapeutics in glioma". PLOS ONE. 8 (5): e62852. Bibcode:2013PLoSO...862852A. doi:10.1371/journal.pone.0062852. PMC 3656086. PMID 23696788.
- ↑ Fuchs M, Wang H, Ciossek T, Chen Z, Ullrich A (1998). "Differential expression of MAM-subfamily protein tyrosine phosphatases during mouse development". Mech Dev. 70 (1–2): 91–109. doi:10.1016/S0925-4773(97)00179-2. PMID 9510027. S2CID 9560178.
- ↑ Horvat-Bröcker A, Reinhard J, Illes S, Paech T, Zoidl G, Harroch S, Distler C, Knyazev P, Ullrich A, Faissner A (2008). "Receptor protein tyrosine phosphatases are expressed by cycling retinal progenitor cells and involved in neuronal development of mouse retina". Neuroscience. 152 (3): 618–45. doi:10.1016/j.neuroscience.2008.01.016. PMID 18308476. S2CID 21471047.
- ↑ Reinhard J, Horvat-Bröcker A, Illes S, Zaremba A, Knyazev P, Ullrich A, Faissner A (2009). "Protein tyrosine phosphatases expression during development of mouse superior colliculus". Exp Brain Res. 199 (3–4): 279–97. doi:10.1007/s00221-009-1963-6. PMC 2845883. PMID 19727691.
Further reading
- Lu J, Li Q, Donadel G, Notkins AL, Lan MS (1998). "Profile and differential expression of protein tyrosine phosphatases in mouse pancreatic islet tumor cell lines". Pancreas. 16 (4): 515–20. doi:10.1097/00006676-199805000-00010. PMID 9598814. S2CID 35807441.
- Bondar C, Plaza-Izurieta L, Fernandez-Jimenez N, Irastorza I, Withoff S, Wijmenga C, Chirdo F, Bilbao JR (2013). "THEMIS and PTPRK in celiac intestinal mucosa: coexpression in disease and after in vitro gliadin challenge". Eur J Hum Genet. 22 (3): 358–62. doi:10.1038/ejhg.2013.136. PMC 3925264. PMID 23820479.