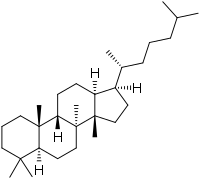
 | |
| Names | |
|---|---|
| IUPAC name
Protostane[1] | |
| Systematic IUPAC name
(1R,3aS,3bS,5aS,9aS,9bS,11aS)-3a,3b,6,6,9a-Pentamethyl-1-[(2R)-6-methylheptan-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C30H54 | |
| Molar mass | 414.762 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Protostane is a tetracyclic triterpene, its natural distribution is primarily limited to the genus Alisma. It is so named because it is considered to be the "prototype" of steroids.[2]
See also
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1539. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Zhao M, Gödecke T, Gunn J, Duan JA, Che CT (2013). "Protostane and Fusidane Triterpenes: A Mini-Review". Molecules. 18 (4): 4054–4080. doi:10.3390/molecules18044054. PMC 3901436. PMID 23563857.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.