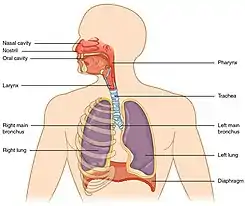
Pulmonary drug delivery is a route of administration in which patients use an inhaler to inhale their medications and drugs are absorbed into the bloodstream via the lung mucous membrane. This technique is most commonly used in the treatment of lung diseases, for example, asthma and chronic obstructive pulmonary disease (COPD). Different types of inhalers include metered-dose inhalers (MDI), dry powder inhalers (DPI), soft mist inhalers (SMI) and nebulizers. The rate and efficacy of pulmonary drug delivery are affected by drug particle properties, breathing patterns and respiratory tract geometry.
Pulmonary drug delivery minimizes systemic side effects and increases bioavailability owing to the localised absorption through the lung. The disadvantages include possible drug irritation to the lung, limited drug dissolution, relatively high drug clearance, and the drug effectiveness depends on the inhaler techniques and patients' compliance. Drug formulation can be challenging since the drug has to bypass the defence mechanisms in the respiratory tract. Pharmacokinetics and pharmacodynamics of the drug in elderly patients can also be particularly difficult to be predicted due to age-related changes in body composition.
Ongoing developments in inhaler device engineering, technology and drug formulations may improve the efficacy and overcome the challenges of pulmonary drug delivery. Recent advancements involve the utilization of the pulmonary route as an entry to systemic circulation for treating different diseases, as well as the development of pulmonary drug formulation and particle engineering technology to increase the efficacy of pulmonary delivery.
Application / Clinical use
Pulmonary drug delivery is mainly utilized for topical applications in the lungs, such as the use of inhaled beta-agonists, corticosteroids and anticholinergic agents for the treatment of asthma and COPD, the use of inhaled mucolytics and antibiotics for the treatment of cystic fibrosis (CT) and respiratory viral infections,[1] and the use of inhaled prostacyclin analogs for the treatment of pulmonary arterial hypertension (PAH).[2]
In addition, this technique is employed for systemic application, for example the use of inhaled insulin for diabetes management,[3] the use of inhaled loxapine for treatment of psychiatric disorders. Vaccines, such as the measles-rubella vaccines, can also be delivered via inhalation.
Examples of inhalers

Metered-dose inhalers (MDIs)
Metered-dose inhalers include pressurized metered-dose inhalers (pMDIs) and breath-actuated metered-dose inhalers (BAMDIs). pMDIs are the most commonly used inhalers for treating lung diseases. It requires coordination of patients’ inhalation and inhaler actuation. BAMDIs are triggered by patients’ inspiratory flow instead of hand actuation, solving the coordination issue.[4] MDIs with spacers have similar effectiveness in drug delivery compared to nebulizers, with additional benefits in convenience and cost-effectiveness.[5] The use of MDIs together with spacers, valved holding chambers (VHCs) or masks improve the efficacy of drug delivery into the lungs.[6]
Advantages
- High portability
- Fixed dose is delivered
- Efficient aerosolized delivery of drug particles
- Inexpensive
- Convenient usage
- Quiet administration[7]
Disadvantages
- Require coordination between inhalation and inhaler actuation[7]
Examples
- Pressurized metered-dose inhalers (pMDIs)
- Breath-actuated metered-dose inhalers (BAMDIs)

Dry powder inhalers (DPIs)
The solid drug powders in DPIs are released by the force of the patient's inspiratory flow. Turbulent airflow generated inside the inhaler by the inhalation force is associated with the movement of airflow and the resistance inside the inhaler.[8] Patients should inhale with adequate inspiratory flow to overcome the resistance of DPIs, leading to drug particle deaggregation for successful pulmonary delivery.[9]
Advantages
Disadvantages
Examples
- Turbuhaler
- Accuhaler
- Handihaler
- Genuair
- Ellipta
- Breexhaler

Soft-mist inhalers (SMIs)
Soft-mist inhaler aerosolized a fixed dose of liquid drug formulation into inhalable tiny particles through an extremely fine nozzle system using the energy generated by the lever-compressed spring, without the use of propellants.[11] The slow and prolonged duration of aerosolization facilitates the patient's coordination between inhaler actuation and inhalation.[12]
Advantages
- High portability
- Require minimal patient's coordination between inhalation and inhaler actuation
- High drug deposition in lungs[13]
Disadvantages
- Low availability in markets
- Relatively expensive[10]
Example
- Respimat
Nebulizers
Nebulizer is mainly used in emergencies, or by patients with poor compliance to other handy inhalers. Nebulizer delivers medication into the lungs by converting water-based liquid drug formulations into inhalable droplets mechanically, such as the use of an ultrasonic system, or thermally.[14] Major types of nebulizers include vibrating mesh nebulizers (VMN), jet nebulizers (JN) and ultrasonic nebulizers.[15]
Advantages
- Patients' education and coordination are not required.
- Suitable for older patients and children
- Better patient satisfaction due to the visible aerosols
- Easily employed with tidal breathing[16]
Disadvantages
- Long time of drug delivery
- Inaccurate drug dosages
- Bulky device
- Expensive
- Require regular maintenance
- Low drug delivery efficiency to the lungs[16]
Examples
- Vibrating mesh nebulizers (VMN)
- Jet nebulizers (JN)
- Ultrasound nebulizers
Factors affecting pulmonary drug delivery
To achieve successful pulmonary drug delivery, a fraction of the inhaled particles should not deposit on the upper respiratory tract since they will be swallowed or expectorated without reaching the lungs, leading to the loss of pharmacological effect or provoking unwanted systemic side effects. Factors affecting the deposition of drug particles in lungs include drug particle properties, breathing patterns and respiratory tract geometry.[17]
Drug particle properties
Particle diameter and particle density significantly affect the drug deposition pattern in the respiratory tract, and are the most common considerations for formulation of pulmonary drugs. Drug particles with diameter larger than 5 μm, predominantly deposit on the upper respiratory tract, limiting the amount of drug particles reaching the lung. Moderate-size drug particles with diameter between 2 μm to 5 μm, primarily deposit on the central and small airways. Small drug particles with diameter smaller than 2 μm, predominantly deposit on the alveolar sacs.[18] Other factors affecting deposition of drugs include particle electrostatic charge, particle shape and particle volatility. Electrostatic charge of the drug particles enhances deposition due to the formation of electrostatic force on the wall of the respiratory tract. Non-spherical particle shape has a different entry pathway compared to that of the spherical particles, causing a change in deposition pattern. Particle volatility affects particle diameter due to the change of particle diameter during condensation and evaporation.[19]
Breathing patterns
Drug particle deposition is associated with mean residence time and tidal volume. An increase in mean residence time or tidal volume enhances drug deposition in lungs, while an increase in air flow decreases the mean residence time, resulting in the decrease of total deposition of drug particles.[20]
Respiratory tract geometry
The bifurcation of trachea into bronchi with smaller diameter increases turbulent flow, leading to an increase in deposition in the large respiratory tract by impaction.[20]
Advantages
Several advantages are associated with the pulmonary route of administration. For respiratory diseases, drug can be delivered directly to the disease site to perform topical relief, thus rapid onset of action can be achieved and there is less systemic side effects.[21] Less dosage of drug can also achieve similar therapeutic effect compared to other routes of administration. For drugs designed to exert systemic effect through the lung as a drug target, the drug can reach the circulation bypassing poor gastrointestinal absorption and hepatic first pass metabolism which improve drug bioavailability.[21] The large absorptive surface area, highly permeable membrane with rich blood supply also enable rapid onset of action and increase bioavailability of the drug.[21]
Disadvantages and challenges
Despite a number of advantages in the pulmonary route compared to other routes of administration, numerous disadvantages are associated with the pulmonary route. As the drug needs to be delivered through the respiratory tract to the lungs, drug formulation can be challenging due to the defense mechanisms which intend to remove or inactivate the exogenous chemicals. Airway constriction and mucus secretion with ciliary movement prevent drugs from reaching the lungs, while enzymes, macrophages and surfactant in the lungs may also inactivate the drugs leading to less drug being absorbed.[2] Studies show that only around 20% of drug reaches the lung for each inhalation and drug loss is mainly due to the accumulation in the oropharynx in terms of pMDIs and DPIs and drug retention in the device for nebulisers.[2]
Some irritating drug particles may also cause local side effects at the respiratory tract, for example inhaled corticosteroid accumulating in the oropharynx can result in dysphonia and oral thrush. Besides, drug dosing may be inaccurate due to the variations of breathing patterns between individuals and the presence of numerous factors affecting the deposition and absorption of drug particles in the lungs.[20] In particular, elder patients may not have enough strength to generate sufficient inspiratory flow, resulting in less drug inhalation and hence low drug bioavailability. Finally, inhalers, especially nebulizers, require regular maintenance and cleaning. The inhaler devices are relatively expensive compared to oral tablets,[22] which may not be affordable to low income patients.
The effectiveness of drug delivery highly depends on the patient's compliance and proper inhaler technique with no significant error in using the inhalers. Poor compliance may lead to uncontrolled or poorly controlled disease status.[23] For instance, a patient may feel recovered and discontinue the treatment, or a patient may forget to take the medication, resulting in suboptimal disease management. Reducing the amount of puffs by combination inhalers delivering two or more drugs in one breath or the use of electronic data loggers can improve compliance.[23]
Incorrect inhaler techniques, such as poor coordination, no exhalation before inhaling the drug aerosol or not holding breath for a few seconds after inhalation may lead to medication depositing inside the respiratory tract instead of the lungs, resulting in inefficient and inadequate treatment.[23][24] Practical demonstration instead of verbal instruction, education and rechecking on the inhaler technique after a period of time can reduce error and enhance true compliance.
Ongoing development
The use of the pulmonary route as an entry into the systemic circulation is constantly developing due to the additional benefits of bypassing the hepatic first pass metabolism, rapid systemic absorption, higher patients compliance and its non-invasive nature. Potent drugs with the ability to penetrate the lung mucosa into the blood circulation may be available for treating diseases requiring systemic drug delivery.[25] The ongoing researches include the use of inhaled nicotine for smoking cessation,[26] the use of inhaled levodopa for the treatment of Parkinson's disease,[27] and the pulmonary delivery of various biologics.[28]
In addition to the development of new pulmonary drugs, the drug formulation and particle engineering technology is advancing, such as the use of Ultrasound Mediated Amorphous to Crystalline transition (UMAX) process to micronize drug into inhalable drug particles with better performance,[29] the use of drug nanoparticles to minimize unwanted drug adverse effects and increase drug bioavailability at the target site,[30] and the use of porous drug particles to improve pulmonary delivery efficacy.[31]
See also
References
- ↑ Liang, Wanling; Pan, Harry W.; Vllasaliu, Driton; Lam, Jenny K. W. (2020-10-26). "Pulmonary Delivery of Biological Drugs". Pharmaceutics. 12 (11): 1025. doi:10.3390/pharmaceutics12111025. ISSN 1999-4923. PMC 7693150. PMID 33114726.
- 1 2 3 Newman, Stephen P (2017). "Drug delivery to the lungs: challenges and opportunities". Therapeutic Delivery. 8 (8): 647–661. doi:10.4155/tde-2017-0037. ISSN 2041-5990. PMID 28730933.
- ↑ Chan, Jason; Cheng-Lai, Angela (2017). "Inhaled Insulin: A Clinical and Historical Review". Cardiology in Review. 25 (3): 140–146. doi:10.1097/CRD.0000000000000143. ISSN 1061-5377. PMID 28379903. S2CID 22883456.
- ↑ Cataldo, Didier; Hanon, Shane; Peché, Rudi V.; Schuermans, Daniel J.; Degryse, Jean M.; De Wulf, Isabelle A.; Elinck, Karin; Leys, Mathias H.; Rummens, Peter L.; Derom, Eric (2022). "How to Choose the Right Inhaler Using a Patient-Centric Approach?". Advances in Therapy. 39 (3): 1149–1163. doi:10.1007/s12325-021-02034-9. ISSN 0741-238X. PMC 8790222. PMID 35080761.
- ↑ Roncada, Cristian; Andrade, Julia; Bischoff, Luísa Carolina; Pitrez, Paulo Márcio (2018-07-10). "COMPARAÇÃO DE DUAS TÉCNICAS INALATÓRIAS PARA ADMINISTRAR BRONCODILATADOR EM CRIANÇAS E ADOLESCENTES COM CRISE AGUDA DE ASMA: METANÁLISE". Revista Paulista de Pediatria. 36 (3): 364–371. doi:10.1590/1984-0462/;2018;36;3;00002. ISSN 1984-0462. PMC 6202895. PMID 29995144.
- ↑ Volerman, Anna; Balachandran, Uma; Siros, Michelle; Akel, Mary; Press, Valerie G. (2021). "Mask Use with Spacers/Valved Holding Chambers and Metered Dose Inhalers among Children with Asthma". Annals of the American Thoracic Society. 18 (1): 17–22. doi:10.1513/AnnalsATS.202005-522CME. ISSN 2329-6933. PMC 7780969. PMID 33052700.
- 1 2 3 4 Chandel, Akshay; Goyal, Amit K.; Ghosh, Goutam; Rath, Goutam (2019). "Recent advances in aerosolised drug delivery". Biomedicine & Pharmacotherapy. 112: 108601. doi:10.1016/j.biopha.2019.108601. PMID 30780107. S2CID 73456189.
- ↑ Lavorini, Federico; Chudek, Jerzy; Gálffy, Gabriella; Pallarés-Sanmartin, Abel; Pelkonen, Anna S.; Rytilä, Paula; Syk, Jörgen; Szilasi, Maria; Tamási, Lilla; Xanthopoulos, Athanasios; Haahtela, Tari (2021-12-01). "Switching to the Dry-Powder Inhaler Easyhaler®: A Narrative Review of the Evidence". Pulmonary Therapy. 7 (2): 409–427. doi:10.1007/s41030-021-00174-5. ISSN 2364-1746. PMC 8477976. PMID 34581994.
- ↑ Ghosh, Sohini; Ohar, Jill A.; Drummond, M. Bradley (2017). "Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: Implications for Dry Powder Inhalers". Journal of Aerosol Medicine and Pulmonary Drug Delivery. 30 (6): 381–387. doi:10.1089/jamp.2017.1416. ISSN 1941-2711. PMC 5915227. PMID 28933581.
- 1 2 3 ElKasabgy, Nermeen A.; Adel, Islam M.; Elmeligy, Mohamed F. (2020-08-21). "Respiratory Tract: Structure and Attractions for Drug Delivery Using Dry Powder Inhalers". AAPS PharmSciTech. 21 (7): 238. doi:10.1208/s12249-020-01757-2. ISSN 1530-9932. PMID 32827062. S2CID 221218042.
- ↑ Iwanaga, Takashi; Tohda, Yuji; Nakamura, Shuhei; Suga, Yasunori (2019-11-01). "The Respimat® Soft Mist Inhaler: Implications of Drug Delivery Characteristics for Patients". Clinical Drug Investigation. 39 (11): 1021–1030. doi:10.1007/s40261-019-00835-z. ISSN 1179-1918. PMC 6800401. PMID 31377981.
- ↑ Wachtel, Herbert; Kattenbeck, Sabine; Dunne, Stephen; Disse, Bernd (2017). "The Respimat® Development Story: Patient-Centered Innovation". Pulmonary Therapy. 3 (1): 19–30. doi:10.1007/s41030-017-0040-8. ISSN 2364-1754. S2CID 51800400.
- ↑ Sorino, Claudio; Negri, Stefano; Spanevello, Antonio; Visca, Dina; Scichilone, Nicola (2020-05-01). "Inhalation therapy devices for the treatment of obstructive lung diseases: the history of inhalers towards the ideal inhaler". European Journal of Internal Medicine. 75: 15–18. doi:10.1016/j.ejim.2020.02.023. ISSN 0953-6205. PMID 32113944. S2CID 211727980.
- ↑ Longest, Worth; Spence, Benjamin; Hindle, Michael (2019-10-01). "Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs". Journal of Aerosol Medicine and Pulmonary Drug Delivery. 32 (5): 317–339. doi:10.1089/jamp.2018.1508. ISSN 1941-2711. PMC 6781258. PMID 31287369.
- ↑ McCarthy, Sean D.; González, Héctor E.; Higgins, Brendan D. (2020). "Future Trends in Nebulized Therapies for Pulmonary Disease". Journal of Personalized Medicine. 10 (2): 37. doi:10.3390/jpm10020037. ISSN 2075-4426. PMC 7354528. PMID 32397615.
- 1 2 Khairnar, Sakshi V; Jain, Divya D; Tambe, Srushti M; Chavan, Yashashri R; Amin, Purnima D (2022). "Nebulizer systems: a new frontier for therapeutics and targeted delivery". Therapeutic Delivery. 13 (1): 31–49. doi:10.4155/tde-2021-0070. ISSN 2041-5990. PMID 34766509. S2CID 244041247.
- ↑ Martin, Andrew R.; Moore, Charles P.; Finlay, Warren H. (2018-12-02). "Models of deposition, pharmacokinetics, and intersubject variability in respiratory drug delivery". Expert Opinion on Drug Delivery. 15 (12): 1175–1188. doi:10.1080/17425247.2018.1544616. ISSN 1742-5247. PMID 30388902. S2CID 53266146.
- ↑ Darquenne, Chantal (2020-08-01). "Deposition Mechanisms". Journal of Aerosol Medicine and Pulmonary Drug Delivery. 33 (4): 181–185. doi:10.1089/jamp.2020.29029.cd. ISSN 1941-2711. PMID 32598200. S2CID 220275931.
- ↑ Finlay, Warren H. (2021-08-01). "Deposition of Aerosols in the Lungs: Particle Characteristics". Journal of Aerosol Medicine and Pulmonary Drug Delivery. 34 (4): 213–216. doi:10.1089/jamp.2021.29040.whf. ISSN 1941-2711. PMID 34264776. S2CID 235959447.
- 1 2 3 Rangaraj, Nagarjun; Pailla, Sravanthi Reddy; Sampathi, Sunitha (2019). "Insight into pulmonary drug delivery: Mechanism of drug deposition to device characterization and regulatory requirements". Pulmonary Pharmacology & Therapeutics. 54: 1–21. doi:10.1016/j.pupt.2018.11.004. PMID 30447295. S2CID 53670288.
- 1 2 3 Labiris, N. R.; Dolovich, M. B. (2003). "Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications: Physiological factors affecting the effectiveness of inhaled drugs". British Journal of Clinical Pharmacology. 56 (6): 588–599. doi:10.1046/j.1365-2125.2003.01892.x. PMC 1884307. PMID 14616418.
- ↑ Khan, Muhammad Suleman; Roberts, Michael S. (2018). "Challenges and innovations of drug delivery in older age". Advanced Drug Delivery Reviews. 135: 3–38. doi:10.1016/j.addr.2018.09.003. PMID 30217519. S2CID 52276293.
- 1 2 3 Chrystyn, Henry; van der Palen, Job; Sharma, Raj; Barnes, Neil; Delafont, Bruno; Mahajan, Anadi; Thomas, Mike (2017). "Device errors in asthma and COPD: systematic literature review and meta-analysis". npj Primary Care Respiratory Medicine. 27 (1): 22. doi:10.1038/s41533-017-0016-z. ISSN 2055-1010. PMC 5434773. PMID 28373682.
- ↑ Melani, Andrea S. (2021). "Inhaler technique in asthma and COPD: challenges and unmet knowledge that can contribute to suboptimal use in real life". Expert Review of Clinical Pharmacology. 14 (8): 991–1003. doi:10.1080/17512433.2021.1929922. ISSN 1751-2433. PMID 33983092. S2CID 234486685.
- ↑ Hickey, Anthony J. (2020). "Emerging trends in inhaled drug delivery". Advanced Drug Delivery Reviews. 157: 63–70. doi:10.1016/j.addr.2020.07.006. PMC 7354278. PMID 32663488.
- ↑ Jackson, Asti; Grobman, Ben; Krishnan-Sarin, Suchitra (2020). "Recent findings in the pharmacology of inhaled nicotine: Preclinical and clinical in vivo studies". Neuropharmacology. 176: 108218. doi:10.1016/j.neuropharm.2020.108218. PMC 7529934. PMID 32592708.
- ↑ Paik, Julia (2020). "Levodopa Inhalation Powder: A Review in Parkinson's Disease". Drugs. 80 (8): 821–828. doi:10.1007/s40265-020-01307-x. ISSN 0012-6667. PMID 32319076. S2CID 216033034.
- ↑ Anselmo, Aaron C.; Gokarn, Yatin; Mitragotri, Samir (2019). "Non-invasive delivery strategies for biologics". Nature Reviews Drug Discovery. 18 (1): 19–40. doi:10.1038/nrd.2018.183. ISSN 1474-1776. PMID 30498202. S2CID 54166853.
- ↑ Biddiscombe, Martyn F.; Usmani, Omar S. (2018). "Is there room for further innovation in inhaled therapy for airways disease?". Breathe. 14 (3): 216–224. doi:10.1183/20734735.020318. ISSN 1810-6838. PMC 6118889. PMID 30186519.
- ↑ García-Fernández, Alba; Sancenón, Félix; Martínez-Máñez, Ramón (2021). "Mesoporous silica nanoparticles for pulmonary drug delivery". Advanced Drug Delivery Reviews. 177: 113953. doi:10.1016/j.addr.2021.113953. PMID 34474094.
- ↑ Duong, Thoa; López-Iglesias, Clara; Szewczyk, Piotr K.; Stachewicz, Urszula; Barros, Joana; Alvarez-Lorenzo, Carmen; Alnaief, Mohammad; García-González, Carlos A. (2021-05-04). "A Pathway From Porous Particle Technology Toward Tailoring Aerogels for Pulmonary Drug Administration". Frontiers in Bioengineering and Biotechnology. 9: 671381. doi:10.3389/fbioe.2021.671381. ISSN 2296-4185. PMC 8129550. PMID 34017828.