 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.014.984 |
| Chemical and physical data | |
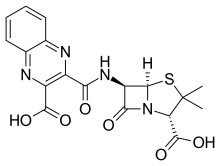
| Formula | C18H16N4O6S |
| Molar mass | 416.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Quinacillin is a penicillin antibiotic which can reversibly deactivate beta-lactamase enzymes.[1] Activity against Staphylococcus aureus is much more potent than against other Gram-positive organisms.[2]
References
- ↑ Persaud KC, Pain RH, Virden R (August 1986). "Reversible deactivation of beta-lactamase by quinacillin. Extent of the conformational change in the isolated transitory complex". The Biochemical Journal. 237 (3): 723–30. doi:10.1042/bj2370723. PMC 1147050. PMID 3492197.
- ↑ Hugo WB, Stretton RG (June 1964). "Action of Quinacillin on Staphylococcus aureus". Nature. 202 (4938): 1217. Bibcode:1964Natur.202.1217H. doi:10.1038/2021217a0. PMID 14217514. S2CID 4198059.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.