 | |
| Names | |
|---|---|
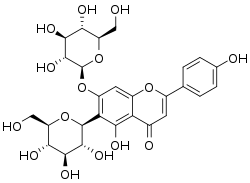
| IUPAC name
5-Hydroxy-6-(β-D-glucopyranosyl)-7-(β-D-glucopyranosyloxy)flavone | |
| Systematic IUPAC name
5-Hydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Isovitexin-7-O-glucoside Saponaretin-7-O-glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H30O15 | |
| Molar mass | 594.52 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Saponarin is a flavone glucoside. It is found in Saponaria officinalis and in Strongylodon macrobotrys where it imparts the characteristic jade color to the flower. This coloration has been shown to be an example of copigmentation, a result of the presence of malvin (an anthocyanin) and saponarin in the ratio 1:9. Under the alkaline conditions (pH 7.9) found in the sap of the epidermal cells, this combination produced a blue-green pigmentation; the pH of the colorless inner floral tissue was found to be lower, at pH 5.6. Experiments showed that saponarin produced a strong yellow colouring in slightly alkaline conditions, resulting in the greenish tone of the flower.[1] It is also found in passion flowers (Passiflora sp.).
References
- ↑ Greenish blue flower colour of Strongylodon macrobotrys. Kosaku Takeda, Aki Fujii, Yohko Senda and Tsukasa Iwashina, Biochemical Systematics and Ecology, Volume 38, Issue 4, August 2010, Pages 630–633, doi:10.1016/j.bse.2010.07.014
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.