A smart cosubstrate is a type of cosubstrate used for cofactor regeneration to yield greater productivity and lower environmental impact (E-factor). A good example of a smart cosubstrate is a lactonizable diol.
In redox biocatalysis, the nicotinamide cofactor (NAD(P)H or NAD(P)+) can act as an electron donor or acceptor by releasing or accepting a hydride. The cofactor must be used in the reaction either in stoichiometric amounts leading to inhibition and economic issues, or in catalytic amounts coupled with an in situ regeneration system. A common approach catalytic amounts is excess use of sacrificial organic molecules such as isopropanol or ethanol. This approach, however, leads to stoichiometric amounts of waste.
The use of 1,4-butanediol as a smart cosubstrate for cofactor regeneration was the next step towards more sustainable redox biocatalysis (Scheme 1).[1] The formation of a thermodynamically stable gamma-butyrolactone as a co-product drives the reaction to completion while yielding higher reaction rates. The use of 1,4-butanediol as an intelligent cosubstrate has also been validated in non-aqueous media using a commercial ADH.[2][3]
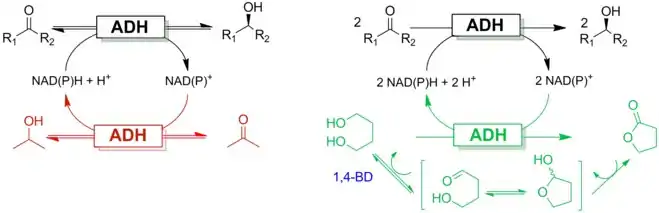
Double-smart cosubstrate
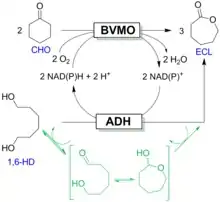
Biocatalytic cascade reactions currently fall into four different categories:
However, only two types of redox-neutral cascades have been reported for the in situ regeneration of the cofactors: parallel cascades (i.e., bi-substrate — no intermediate — bi- or tri-product) and linear cascades (i.e., single substrate — single intermediate — single product).[6][7][8]
The concept of a smart cosubstrate was developed further through the design of a new class of redox-neutral "convergent cascade" reactions. Convergent cascade reactions involve a bi-substrate and a single product without the formation of an intermediate and were developed for the production of epsilon-caprolactone, which consists of a Baeyer-Villiger monooxygenase; for the oxidation of cyclohexanone; an alcohol dehydrogenase for oxidation of the "double-smart cosubstrate" 1,6-hexanediol; and for simultaneous regeneration of the nicotinamide cofactor.[9] In 2016, two-step optimization of the convergent cascade by Design-of-Experiments and a biphasic system was reported.[10]
Smart cosubstrates are an elegant solution for thermodynamically limited redox reactions and have many advantages:
- Less conventional cosubstrates (e.g., isopropanol, ethanol) that negatively affect the enzymes’ activities need to be used.
- Less waste is generated.
- Reactions are faster, which could be caused by the absence of acetone or acetaldehyde as a coproduct, which lead to reduced enzyme activities.
References
- ↑ Kara S, Spickermann D, Schrittwieser JH, Leggewie C, van Berkel WJH, Arends IWCE, Hollmann F (2013) More efficient redox biocatalysis by utilising 1,4-butanediol as a "smart cosubstrate". Green Chem 15 (2):330-335. doi:10.1039/c2gc36797a
- ↑ Kara S, Spickermann D, Weckbecker A, Leggewie C, Arends IWCE, Hollmann F (2014) Bioreductions Catalyzed by an Alcohol Dehydrogenase in Non-aqueous Media. ChemCatChem 6 (4):973-976. doi:10.1002/cctc.201300841
- ↑ Zuhse R, Leggewie C, Hollmann F, Kara S (2015) Scaling-Up of “Smart Cosubstrate” 1,4-Butanediol Promoted Asymmetric Reduction of Ethyl-4,4,4-trifluoroacetoacetate in Organic Media. Org Process Res Dev 19 (2):369-372. doi:10.1021/op500374x
- ↑ García-Junceda E, Lavandera I, Rother D, Schrittwieser JH (2015) (Chemo)enzymatic cascades—Nature's synthetic strategy transferred to the laboratory. J Mol Catal B: Enzym 114 (0):1-6. doi:https://dx.doi.org/10.1016/j.molcatb.2014.12.007
- ↑ Schrittwieser JH, Sattler J, Resch V, Mutti FG, Kroutil W (2011) Recent biocatalytic oxidation-reduction cascades. Curr Opin Chem Biol 15 (2):249-256. doi:10.1016/j.cbpa.2010.11.010
- ↑ Kara S, Schrittwieser JH, Hollmann F, Ansorge-Schumacher MB (2014) Recent trends and novel concepts in cofactor-dependent biotransformations. Appl Microbiol Biotechnol 98 (4):1517-1529. doi:10.1007/s00253-013-5441-5
- ↑ Kara S, Schrittwieser JH, Hollmann F (2013) Strategies for Cofactor Regeneration in Biocatalyzed Reductions. In: Synthetic Methods for Biologically Active Molecules. Wiley-VCH Verlag GmbH & Co. KGaA, pp 209-238. doi:10.1002/9783527665785.ch08
- ↑ Hummel W, Gröger H (2014) Strategies for regeneration of nicotinamide coenzymes emphasizing self-sufficient closed-loop recycling systems. J Biotechnol 191 (0):22-31. doi:https://dx.doi.org/10.1016/j.jbiotec.2014.07.449
- ↑ Bornadel A, Hatti-Kaul R, Hollmann F, Kara S (2015) A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a ‘Double-Smart Cosubstrate’. ChemCatChem 7 (16):2442-2445. doi:10.1002/cctc.201500511
- ↑ Bornadel A, Hatti-Kaul R, Hollmann F, Kara S (2016) Enhancing the productivity of the bi-enzymatic convergent cascade for ɛ-caprolactone synthesis through the design of experiments and a biphasic system. Tetrahedron 72:7222-7228 doi:https://dx.doi.org/10.1016/j.tet.2015.11.054