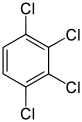
 1,2,3,4-Tetrachlorobenzene | |
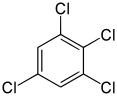 1,2,3,5-Tetrachlorobenzene | |
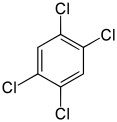 1,2,45-Tetrachlorobenzene | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.032.390 |
| EC Number |
|
| KEGG |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H2Cl4 | |
| Molar mass | 215.88 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tetrachlorobenzene is any of three isomeric chlorobenzenes with the molecular formula C6H2Cl4. They differ by the positions of the chlorine atoms around the ring. Tetrachlorobenzenes are colorless crystalline compounds.[1]
Properties
| isomer | m.p. (°C) | b.p. (°C) | m.p. (g/cm3 @100 °C) |
|---|---|---|---|
| 1,2,3,4 | 47 | 254 | 1.539 |
| 1,2,3,5 | 51.5 | 246 | 1.523 |
| 1,2,4,5 | 141 | 245 | 1.454 |
Synthesis
1,2,4,5-Tetrachlorobenzene can be produced by electrophilic halogenation of benzenes and some chlorobenzenes.[2] 1,2,3,4-Tetrachlorobenzene can only be produced by chlorination of 1,3,5-trichlorobenzene.
Uses
1,2,4,5-tetrachlorobenzene once was used as intermediates in the production of pesicides,[3] specifically trichlorophenols. This method has been discontinued because it also produced 2,3,7,8-tetrachlorodibenzo-p-dioxin.[1]
See also
- Chlorobenzenes—different numbers of chlorine substituents
References
- 1 2 Beck, Uwe; Löser, Eckhard (2011). "Chlorinated Benzenes and Other Nucleus‐Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.o06_o03. ISBN 9783527303854.
- ↑ "US4205014A Process for 1,2,4,5-tetrachlorobenzene". Espacenet. Retrieved 22 June 2023.
- ↑ NTP Toxicity Study Reports. National Toxicology Program. 1991. p. 11. Retrieved 22 June 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.