 | |
| Clinical data | |
|---|---|
| Trade names | Vyzulta |
| Other names | BOL-303259-X |
| AHFS/Drugs.com | Multum Consumer Information |
| License data | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.251.571 |
| Chemical and physical data | |
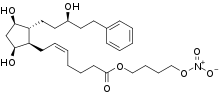
| Formula | C27H41NO8 |
| Molar mass | 507.624 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Latanoprostene bunod (trade name Vyzulta) is an ophthalmic drug approved in the United States in 2017 for the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.[3][4] It targets the trabecular meshwork directly.[4]
References
- ↑ "Search Page - Drug and Health Product Register". 23 October 2014.
- ↑ "Vyzulta- latanoprostene bunod solution/ drops". DailyMed. 1 May 2019. Retrieved 7 June 2022.
- ↑ "FDA Approves Vyzulta (latanoprostene bunod) Ophthalmic Solution for Open-Angle Glaucoma, Ocular Hypertension" (Press release). Valeant Pharmaceuticals International, Inc.
- 1 2 Hoy SM (May 2018). "Latanoprostene Bunod Ophthalmic Solution 0.024%: A Review in Open-Angle Glaucoma and Ocular Hypertension". Drugs. 78 (7): 773–780. doi:10.1007/s40265-018-0914-6. PMC 5976683. PMID 29761382.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.