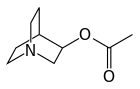 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical (ophthalmic solution) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | deacetylation? |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.431 |
| Chemical and physical data | |
| Formula | C9H15NO2 |
| Molar mass | 169.224 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Aceclidine (Glaucostat, Glaunorm, Glaudin) is a parasympathomimetic miotic agent used in the treatment of narrow angle glaucoma.
Medicinal properties
Aceclidine decreases intraocular pressure. It acts as a muscarinic acetylcholine receptor agonist.[1]
Side effects of aceclidine include increased salivation and bradycardia (in excessive doses).
Chemistry
Aceclidine is an organic compound that is structurally related to quinuclidine. As such its alternative name is 3-acetoxyquinuclidine. Its protonated derivative has a pKa of 9.3.[2]
See also
- Talsaclidine (drug with a similar structure)
- Muscarine
References
- ↑ Shannon HE, Hart JC, Bymaster FP, Calligaro DO, DeLapp NW, Mitch CH, et al. (August 1999). "Muscarinic receptor agonists, like dopamine receptor antagonist antipsychotics, inhibit conditioned avoidance response in rats". The Journal of Pharmacology and Experimental Therapeutics. 290 (2): 901–907. PMID 10411607.
- ↑ Aggarwal VK, Emme I, Fulford SY (February 2003). "Correlation between pK(a) and reactivity of quinuclidine-based catalysts in the Baylis-Hillman reaction: discovery of quinuclidine as optimum catalyst leading to substantial enhancement of scope". The Journal of Organic Chemistry. 68 (3): 692–700. doi:10.1021/jo026671s. PMID 12558387.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.