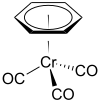
苯三羰基铬
苯三羰基铬是一种金属有机化合物,化学式为Cr(C6H6)(CO)3,它是黄色晶体,可溶于常见的非极性有机溶剂。它最初于1957年由Fischer和Öfele报道,他们通过二苯铬的羰基化反应制备。[1]
- Cr(CO)6 + C6H6 → Cr(C6H6)(CO)3 + 3 CO
| (Benzene)chromium(0) tricarbonyl | |||
|---|---|---|---|
| |||
| IUPAC名 (benzene)tricarbonylchromium | |||
| 别名 | 三羰基(苯)铬 | ||
| 识别 | |||
| CAS号 | 12082-08-5 | ||
| PubChem | 10954891 | ||
| ChemSpider | 9130108 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | WVSBQYMJNMJHIM-UHFFFAOYAR | ||
| 性质 | |||
| 化学式 | Cr(C6H6)(CO)3 | ||
| 214.14 g/mol g·mol⁻¹ | |||
| 外观 | 黄色晶体 | ||
| 熔点 | 163 - 166 °C | ||
| 溶解性(水) | 难溶 | ||
| 溶解性 | THF, 乙醚, 苯 | ||
| 结构 | |||
| 配位几何 | tetrahedral, "piano stool" | ||
| 危险性 | |||
GHS危险性符号 | |||
| GHS提示词 | Warning | ||
| H-术语 | H302, H312, H332 | ||
| P-术语 | P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | ||
| 主要危害 | Harmful through inhalation, contact with skin, or swallowed | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
它的苯环比苯本身更亲电,可以发生亲核加成反应:[2]
其酸性也更强,锂化后可以进行一系列反应:
参考文献
- Fischer, Ernst Otto; Őfele, Karl. (1957). “Über Aromatenkomplexe von Metallen, XIII Benzol-Chrom-Tricarbonyl,” Chemische Berichte, 90, 2532-5. doi:10.1002/cber.19570901117.
- Herndon, James W; Laurent, Stéphane E. (2008). “(η6-Benzene)tricarbonylchromium,” in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Chichester, 2008. doi:10.1002/047084289X.rb025.pub2. Article Online Posting Date: March 15, 2009
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
chromium-tricarbonyl-from-xtal-1987-3D-balls.png.webp)
chromiumtricarbonyl_electrophile_nucleophilic_carbonylation.png.webp)
chromiumtricarbonyl_lithiation_TMS.png.webp)