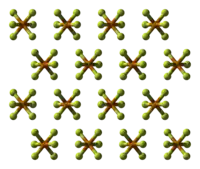六氟化碲
六氟化碲是一种无机化合物,化学式为TeF6。它是无色、高毒的气体,有恶臭气味。
| 六氟化碲 | |||
|---|---|---|---|
| |||
| 英文名 | |||
| 别名 | 氟化碲(VI) | ||
| 识别 | |||
| CAS号 | 7783-80-4 | ||
| PubChem | 24559 | ||
| SMILES |
| ||
| InChI |
| ||
| EINECS | 232-027-0 | ||
| 性质 | |||
| 化学式 | TeF6 | ||
| 241.590 g/mol g·mol⁻¹ | |||
| 外观 | 无色气体 | ||
| 氣味 | 恶臭气味 | ||
| 密度 | 0.0106 g/cm3 (-10 °C) 4.006 g/cm3 (-191 °C) | ||
| 熔点 | −38.9 °C(234.2 K)[1] | ||
| 沸点 | −37.6 °C(236 K) | ||
| 溶解性(水) | 反应 | ||
| 蒸氣壓 | >1 atm (20°C)[2] | ||
| 磁化率 | −66.0·10−6 cm3/mol | ||
| 折光度n D |
1.0009 | ||
| 结构 | |||
| 晶体结构 | 正交晶系,oP28 | ||
| 空间群 | Pnma, No. 62 | ||
| 配位几何 | octahedral (Oh) | ||
| 偶极矩 | 0 | ||
| 热力学 | |||
| ΔfHm⦵298K | -1318 kJ/mol | ||
| 热容 | 117.6 J/(mol K) | ||
| 危险性 | |||
| PEL | TWA 0.02 ppm (0.2 mg/m3)[2] | ||
| 致死量或浓度: | |||
LCLo(最低) |
5 ppm (rat, 4 hr) 5 ppm (mouse, 1 hr) 5 ppm (rabbit, 4 hr) 5 ppm (guinea pig, 4 hr)[3] | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
化学性质
虽然碲与硫同族,但六氟化碲却不像六氟化硫那样惰性。这可能来自于较大的原子半径,使得碲能够在亲核反应中结合8个原子,而硫和硒仅为6个。
六氟化碲和水反应,生成碲酸。六氟化碲在200℃下可以和碲反应。
- TeF6+6 H2O->Te(OH)6+6 HF
参考文献
- CRC Handbook of Chemistry and Physics, 90. Auflage, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-95.
- NIOSH Pocket Guide to Chemical Hazards. . NIOSH.
- . Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 朱文祥. 无机化合物制备手册. 化学工业出版社. pp 747 【XVI-256】六氟化碲
拓展阅读
- W.C. Cooper; Tellurium, Van Nostrand Reinhold Company, New York, USA, 1971.
- K.W. Bagnall; The Chemistry of Selenium, Tellurium and Polonium, Elsevier Publishing, New York, 1966.
- R.T. Sanderson; Chemical Periodicity, Reinhold, New York, USA, 1960.
- N.N. Greenwood and A. Earnshaw; Chemistry of the Elements, 2nd edition, Butterworth, UK, 1997.
- F. A. Cotton, G. Wilkinson, C.A. Murillo, and M. Bochmann; Advanced Inorganic Chemistry, John Wiley & Sons, 1999.
- G.J. Hathaway, N.H. Proctor; Chemical Hazards of the Workplace, 5th edition, Wiley-Interscience, New Jersey, 2004.
- Web Elements (页面存档备份,存于)
- OSHA
- CDC - NIOSH Pocket Guide to Chemical Hazards (页面存档备份,存于)
参见
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.