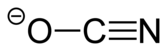氰酸银
氰酸银是一种化合物,氰酸的银盐。它可以通过氰酸钾或尿素与硝酸银的反应来制备。[2]
| 氰酸银 | |||
|---|---|---|---|
| |||
| 系统名 Silver(I) cyanate | |||
| 识别 | |||
| CAS号 | 3315-16-0 | ||
| PubChem | 76827 | ||
| ChemSpider | 69282 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | DWBPIWPCOSHWCK-REWHXWOFAQ | ||
| 性质 | |||
| 化学式 | AgOCN | ||
| 149.885 g/mol g·mol⁻¹ | |||
| 危险性[1] | |||
欧盟危险性符号 有害 Xn 有害 Xn | |||
| 警示术语 | R:R20/21/22 | ||
| 安全术语 | S:S24/25 | ||
GHS危险性符号 | |||
| H-术语 | H302, H312, H332 | ||
| P-术语 | P280 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
银氰酸酯是一种米黄色到灰色的粉末。结晶为单斜,空间群P21/m,其中a = 547.3 pm,b = 637.2 pm,c = 341.6 pm,β = 91°。[3]
参考
- 来源:Sigma-Aldrich Co., product no. {{{id}}} .
- Willy Kühne, [《氰酸银》在Google Books的內容。 Lehrbuch der physiologischen Chemie]. 1868 (德文)
- D. Britton, J. D. Dunitz: The crystal structure of silver cyanate (页面存档备份,存于), Acta Cryst. (1965). 18, 424-428, doi:10.1107/S0365110X65000944
- J. Milbauer: Bestimmung und Trennung der Cyanate, Cyanide, Rhodanide und Sulfide in Fresenius' Journal of Analytical Chemistry 42 (1903) 77-95, doi:10.1007/BF01302741.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.