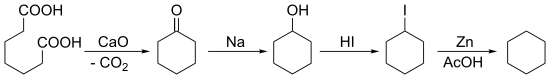
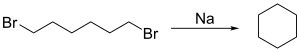
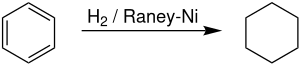环己烷
环己烷,是环烷烃的一种,也称六氢化苯。它是一种无色,易燃,微溶于水,具有挥发性,微有刺激性气味的液体,无腐蚀性,分子式C6H12。
| 环己烷 | |||
|---|---|---|---|
| |||
| |||
| IUPAC名 Cyclohexane[1] | |||
| 英文名 | |||
| 别名 | 六氢化苯[2] | ||
| 识别 | |||
| CAS号 | 110-82-7 | ||
| PubChem | 8078 | ||
| ChemSpider | 7787 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | XDTMQSROBMDMFD-UHFFFAOYAZ | ||
| Beilstein | 1900225 | ||
| Gmelin | 1662 | ||
| 3DMet | B04304 | ||
| UN编号 | 1145 | ||
| ChEBI | 29005 | ||
| RTECS | GU6300000 | ||
| DrugBank | DB03561 | ||
| KEGG | C11249 | ||
| 性质 | |||
| 化学式 | C6H12 | ||
| 摩尔质量 | 84.16 g·mol−1 | ||
| 外观 | 无色液体 | ||
| 氣味 | 甜味,汽油味 | ||
| 密度 | 0.996g·cm-3 | ||
| 密度 | 0.7739g·mL-1 | ||
| 熔点 | 6.47 °C(280 K) | ||
| 沸点 | 80.74 °C(354 K) | ||
| 溶解性(水) | 不混溶 | ||
| 溶解性 | 可溶于乙醚、乙醇和丙酮 | ||
| 蒸氣壓 | 78 mmHg (20 °C)[3] | ||
| 磁化率 | −68.13·10−6 cm3/mol | ||
| 折光度n D |
1.42662 | ||
| 黏度 | 1.02 cP at 17 °C | ||
| 热力学 | |||
| ΔfHm⦵298K | −156 kJ/mol | ||
| ΔcHm⦵ | −3920 kJ/mol | ||
| 危险性 | |||
GHS危险性符号   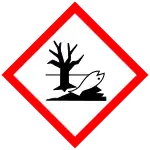 | |||
| GHS提示词 | Danger | ||
| H-术语 | H225, H302, H305, H315, H336 | ||
| P-术语 | P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+310, P302+352, P303+361+353 | ||
| NFPA 704 |
 3
1
0
| ||
| 爆炸極限 | 1.3–8%[3] | ||
| PEL | TWA 300 ppm (1050 mg/m3)[3] | ||
| 致死量或浓度: | |||
LD50(中位剂量) |
12705 mg/kg(大鼠口服) 813 mg/kg(小鼠口服)[4] | ||
LCLo(最低) |
17,142 ppm (小鼠,2 h) 26,600 ppm(兔子,1 h)[4] | ||
| 相关物质 | |||
| 相关环烷烃 | 环戊烷 环庚烷 | ||
| 相关化学品 | 环己烯 苯 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
环己基 (C6H11) 是一种烷基取代基,通常简称为Cy。[5]
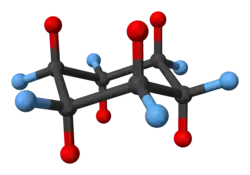
椅型构象的环己烷分子。处于直立键的氢原子标记为红色,处于平伏键的氢原子标记为蓝色。
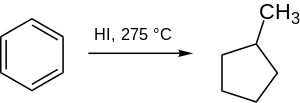
合成
参考资料
- . . Cambridge: The Royal Society of Chemistry. 2014: P001–P004. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069-FP001.
- Hexanaphthene 的存檔,存档日期2018-02-12., dictionary.com
- NIOSH Pocket Guide to Chemical Hazards. . NIOSH.
- . Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- (PDF). The Journal of Organic Chemistry. [2021-07-21]. (原始内容存档 (PDF)于2018-08-01).
- Fred Fan Zhang, Thomas van Rijnman, Ji Soo Kim, Allen Cheng "On Present Methods of Hydrogenation of Aromatic Compounds, 1945 to Present Day" Lunds Tekniska Högskola 2008
- Ceresana. . www.ceresana.com. [4 May 2018]. (原始内容存档于21 December 2017).
- Warnhoff, E. W. . J. Chem. Educ. 1996, 73 (6): 494. Bibcode:1996JChEd..73..494W. doi:10.1021/ed073p494.
- Bertholet (1867) "Nouvelles applications des méthodes de réduction en chimie organique" (页面存档备份,存于) (New applications of reduction methods in organic chemistry), Bulletin de la Société chimique de Paris, series 2, 7 : 53-65.
- Bertholet (1868) "Méthode universelle pour réduire et saturer d'hydrogène les composés organiques" (Universal method for reducing and saturating organic compounds with hydrogen), Bulletin de la Société chimique de Paris, series 2, 9 : 8-31. From page 17: (页面存档备份,存于) "En effet, la benzine, chauffée à 280° pendant 24 heures avec 80 fois son poids d'une solution aqueuse saturée à froid d'acide iodhydrique, se change à peu près entièrement en hydrure d'hexylène, C12H14, en fixant 4 fois son volume d'hydrogène: C12H6 + 4H2 = C12H14 … Le nouveau carbure formé par la benzine est un corps unique et défini: il bout à 69°, et offre toutes les propriétés et la composition de l'hydrure d'hexylène extrait des pétroles." (In effect, benzene, heated to 280° for 24 hours with 80 times its weight of an aqueous solution of cold saturated hydroiodic acid, is changed almost entirely into hydride of hexylene, C12H14, [Note: this formula for hexane (C6H14) is wrong because chemists at that time used the incorrect atomic mass for carbon.] by fixing [i.e., combining with] 4 times its volume of hydrogen: C12H6 + 4H2 = C12H14 … The new carbon compound formed by benzene is a unique and well-defined substance: it boils at 69° and presents all the properties and the composition of hydride of hexylene extracted from oil.)
- Adolf Baeyer (1870) "Ueber die Reduction aromatischer Kohlenwasserstoffe durch Jodphosphonium" (页面存档备份,存于) (On the reduction of aromatic compound by phosphonium iodide [H4IP]), Annalen der Chemie und Pharmacie, 155 : 266-281. From page 279: "Bei der Reduction mit Natriumamalgam oder Jodphosphonium addiren sich im höchsten Falle sechs Atome Wasserstoff, und es entstehen Abkömmlinge, die sich von einem Kohlenwasserstoff C6H12 ableiten. Dieser Kohlenwasserstoff ist aller Wahrscheinlichkeit nach ein geschlossener Ring, da seine Derivate, das Hexahydromesitylen und Hexahydromellithsäure, mit Leichtigkeit wieder in Benzolabkömmlinge übergeführt werden können." (During the reduction [of benzene] with sodium amalgam or phosphonium iodide, six atoms of hydrogen are added in the extreme case, and there arise derivatives, which derive from a hydrocarbon C6H12. This hydrocarbon is in all probability a closed ring, since its derivatives — hexahydromesitylene [1,3,5 - trimethyl cyclohexane] and hexahydromellithic acid [cyclohexane-1,2,3,4,5,6-hexacarboxylic acid] — can be converted with ease again into benzene derivatives.)
- Campbell, M. Larry. . . 2011. ISBN 978-3527306732. doi:10.1002/14356007.a08_209.pub2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.