甲磺酸酐
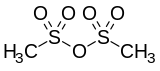
甲磺酸酐是甲磺酸的酸酐。它与甲磺酰氯一样,可用于制备甲磺酰酯。它有市售品,也可以在实验室通过五氧化二磷和甲磺酸在80 °C下的反应制备。[2]
- 2 P2O5 + 6 CH3SO3H → 3 (CH3SO2)2O + 4 H3PO4
| 甲磺酸酐 | |
|---|---|
 | |
 | |
| IUPAC名 methylsulfonyl methanesulfonate | |
| 别名 | 甲基磺酸酐 |
| 识别 | |
| CAS号 | 7143-01-3 |
| PubChem | 81560 |
| ChemSpider | 73591 |
| SMILES |
|
| InChI |
|
| InChIKey | IZDROVVXIHRYMH-UHFFFAOYAS |
| 性质 | |
| 化学式 | C2H6O5S2 |
| 174.20 g/mol g·mol⁻¹ | |
| 外观 | 白色固体 |
| 密度 | 0.92 g/ml[1] |
| 熔点 | 69.5—70 °C(342.6—343.1 K)[2] |
| 溶解性(水) | 水解 |
| 溶解性 | 可溶于非质子有机溶剂 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参见
参考文献
- Wachtmeister, C. A.; Pring, B.; Osterman, Siv; Ehrenberg, L.; Brunvoll, J.; Bunnenberg, E.; Djerassi, Carl; Records, Ruth. . Acta Chemica Scandinavica. 1966, 20: 908–910. doi:10.3891/acta.chem.scand.20-0908.
- Field, Lamar; Settlage, Paul H. . Journal of the American Chemical Society. March 1954, 76 (5): 1222–1225. doi:10.1021/ja01634a005.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.