碲化汞
碲化汞的化学式为HgTe,是由碲和汞元素组成的二元无机化合物。
| 碲化汞 | |
|---|---|
 | |
| IUPAC名 碲化汞(II) | |
| 系统名 碲化汞 | |
| 识别 | |
| CAS号 | 12068-90-5 |
| PubChem | 82914 |
| SMILES |
|
| InChI |
|
| EINECS | 235-108-9 |
| 性质 | |
| 化学式 | HgTe |
| 329.19 g/mol g·mol⁻¹ | |
| 外观 | 近黑色的立方晶体 |
| 密度 | 8.12 g/cm3[1] |
| 溶解性(水) | 不溶于水 |
| 结构 | |
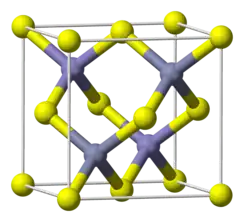
| 晶体结构 | Sphalerite, cF8 |
| 空间群 | F-43m, No. 216 |
| 相关物质 | |
| 其他阴离子 | 氧化汞 硫化汞 硒化汞 |
| 其他阳离子 | 碲化氢 碲化锌 碲化镉 |
| 相关化学品 | 二氧化碲 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考资料
- 《无机化合物制备手册》.朱文祥 主编.化学工业出版社. P388. 【XII-38】碲化汞[mercury(II) telluride]
- Properties of mercury cadmium telluride, Ed. J. Brice and P. Capper, EMIS datareview, (INSPEC, IEE, London, UK, 1987).
- Properties of Narrow-Gap Cadmium-Based Compounds Ed. P. Capper (INSPEC, IEE, London, UK, 1994) ISBN 0-85296-880-9
- Tellurium and Tellurides, D. M. Chizhikov and V. P. Shchastlivyi, 1966, Nauka Publishing, Moscow
- Mercury selenide stoichiometry and phase relations in the mercury-selenium system, N. Z. Boctor and G. Kullerud, Journal of Solid State Chemistry Vol. 62, pp. 177–183 (1986) doi:10.1016/0022-4596(86)90229-X
- Total-energy study of the equation of state of HgTe and HgSe, Z. W. Lu, David Singh, and Henry Krakauer, Phys. Rev. B vol. 39, pp. 10154 (1989).
- Quantum Spin Hall Insulator State in HgTe Quantum Wells, M. Konig et al., Science 318 766 (2007).
拓展阅读
- Thermophysical properties database at Germany's Chemistry Information Centre, Berlin
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.