羟胺
| 羟胺 | |||
|---|---|---|---|
| |||
 | |||
| IUPAC名 hydroxylamine | |||
| 识别 | |||
| CAS号 | 7803-49-8 | ||
| PubChem | 787 | ||
| ChemSpider | 766 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | AVXURJPOCDRRFD-UHFFFAOYAD | ||
| Gmelin | 478 | ||
| 3DMet | B01184 | ||
| EINECS | 232-259-2 | ||
| ChEBI | 15429 | ||
| RTECS | NC2975000 | ||
| KEGG | C00192 | ||
| MeSH | Hydroxylamine | ||
| 性质 | |||
| 化学式 | NH2OH | ||
| 33.0298 g·mol⁻¹ | |||
| 外观 | 白色固体 | ||
| 密度 | 1.21 g/cm3 | ||
| 熔点 | 33 °C | ||
| 沸点 | 110 °C | ||
| 溶解性(水) | 可溶于冷水,热水中分解 | ||
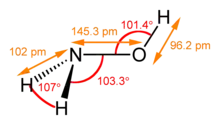
| 结构 | |||
| 偶极矩 | 0.67553 D | ||
| 热力学 | |||
| ΔfHm⦵298K | −39.9 kJ/mol | ||
| S⦵298K | ? J K−1 mol−1 | ||
| 危险性 | |||
| 警示术语 | R:R5, R22, R37/38, R41, R43, R48/22, R50 | ||
| 安全术语 | S:S2, S22, S26, S36/37/39, S61 | ||
| 欧盟分类 | Xn, N | ||
| NFPA 704 |
 3
3
1
| ||
| 相关物质 | |||
| 相关化学品 | 盐酸羟胺、硫酸羟胺 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
1999年来,两家生产羟胺的工厂发生了爆炸,但羟胺的爆炸性原理尚不明确。[2] 研究表明,二价或三价铁盐会催化50%羟胺溶液的分解,羟胺及衍生物最好以盐的形式储存。
羟胺有顺式和反式两种异构体,固态时为反式,气态可能是顺式和反式的混合物。
生产
NH2OH的生产方法有以下几种:
- 0°C下,用HSO4−/SO2还原亚硝酸铵的水溶液,生成[NH4]2[N(OH)(OSO2)2],进一步水解得到硫酸羟胺(NH3OH)2SO4:
- NH4NO2 + 2SO2 + NH3 + H2O → [NH4]2[N(OH)(OSO2)2]
- [NH4]+2[N(OH)(OSO2)2]2− + H2O → [NH4][NH(OH)(OSO2)] + [NH4][HSO4]
- 2[NH4]+[NH(OH)(OSO2)]− + 2H2O → [NH3(OH)]2[SO4] + [NH4]2[SO4]
- HNO2 + 2 HSO3− → [N(OH)(OSO2)2]2− + H2O → [NH(OH)(OSO2)]− + [HSO4]−
- [NH(OH)(OSO2)]− + H3O+ (100 °C/1 h) → [NH3(OH)]+ + [HSO4]−
- [NH3(OH)]Cl + NaOBu → NH2OH + NaCl + BuOH[1]
反应
羟胺与亲电试剂,如烷基化试剂反应生成N或O取代产物:
- R-X + NH2OH → R-ONH2 + HX
- R-X + NH2OH → R-NHOH + HX
- R2C=O + NH2OH∙HCl , NaOH → R2C=NOH + NaCl + H2O
肟通常是具有固定熔点的固体,其生成与分解反应可用于纯化羰基化合物。丁二酮肟等肟类也是常用的配体试剂。
羟胺与氯磺酸反应生成羟胺-O-磺酸,是生产己内酰胺的原料之一:
- HOSO2Cl + NH2OH → NH2OSO2OH + HCl
羟胺-O-磺酸应于0°C储存,使用时也需要用碘量法来测定纯度。
- NH2OH (Zn/HCl) → NH3
- R-NHOH (Zn/HCl) → R-NH2
用途
羟胺及其衍生物的用途有:
安全
羟胺对呼吸系统、皮肤、眼部及黏膜具刺激性,吞食有害,为潜在的诱变剂。[5] 羟胺在加热时可能发生爆炸。
参见
参考资料
- Greenwood and Earnshaw. Chemistry of the Elements. 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431-432. 1997.
- Japan Science and Technology Agency Failure Knowledge Database 的存檔,存档日期2007-12-20..
- Smith, Michael and Jerry March. March's advanced organic chemistry : reactions, mechanisms, and structure. New York. Wiley. p. 1554. 2001.
- Patnaik, Pradyot. Handbook of Inorganic Chemicals. McGraw Hill. pp. 385-386. 2003.
- MSDS Sigma-Aldrich
- Hydroxylamine
- Walters, Michael A. and Andrew B. Hoem. "Hydroxylamine." e-Encyclopedia of Reagents for Organic Synthesis. 2001.
- Schupf Computational Chemistry Lab (页面存档备份,存于)
- M. W. Rathke A. A. Millard "Boranes in Functionalization of Olefins to Amines: 3-Pinanamine" Organic Syntheses, Coll. Vol. 6, p. 943; Vol. 58, p. 32. (preparation of hydroxylamine-O-sulfonic acid).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.