1,2,4-三硝基苯
1,2,4-三硝基苯是一种有机化合物,化学式为C6H3N3O6,它是三硝基苯的同分异构体之一。它可由2,4-二硝基氟苯和亚硝酸钠在18-冠-6的存在下反应得到。[2]它在甲醇中于60 °C可以被含氢氧化钠的硼氢化钠还原,生成1,3-二硝基苯、1,4-二硝基苯和2,4-二硝基苯酚,占比分别为35%、24%、22%。[3]
| 1,2,4-三硝基苯 | |
|---|---|
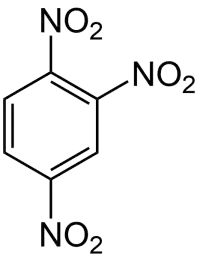 | |
| 识别 | |
| CAS号 | 610-31-1 |
| 性质 | |
| 化学式 | C6H3N3O6 |
| 摩尔质量 | 213.1 g·mol−1 |
| 外观 | 浅黄色固体[1] |
| 熔点 | 60 °C(333 K)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
参考文献
- Borsche, W. β-Nitroarylhydroxylamines. I. β-2,4- and β-2,6-dinitrophenylhydroxylamine. Berichte der Deutschen Chemischen Gesellschaft [Abteilung] B: Abhandlungen, 1923. 56B: 1494-1501. ISSN 0365-9488. CODEN BDCBAD.
- Badea, Florin; Stoica, Alice; Ionita, Petre; Caproiu, Miron T.; Constantinescu, Titus. Nitration of some di- and trinitrohalobenzenes with solid sodium nitrite in the presence of 18-crown-6. Revue Roumaine de Chimie, 2000. 44 (4): 351-356. ISSN 0035-3930. CODEN RRCHAX.
- Dale J, Vikersveen L. The reaction of nitrobenzenes with sodium borohydride; a novel reductive coupling to diphenylamine derivatives. Acta chemica Scandinavica. Series B. Organic chemistry and biochemistry, 1988, 42(6): 354-361.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.