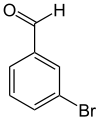
3-溴苯甲醛
3-溴苯甲醛是一种有机化合物,化学式为C7H5BrO。它在室温为无色液体。[6]
| 3-溴苯甲醛 | |
|---|---|
 | |
 | |
| IUPAC名 3-Bromobenzaldehyde | |
| 英文名 | |
| 别名 | 间溴苯甲醛 |
| 识别 | |
| CAS号 | 3132-99-8 |
| ChemSpider | 21106543 |
| SMILES |
|
| InChI |
|
| InChIKey | SUISZCALMBHJQX-UHFFFAOYAJ |
| 性质 | |
| 化学式 | C7H5BrO |
| 摩尔质量 | 185.02 g·mol−1 |
| 外观 | 无色液体 |
| 密度 | 1.587 g·cm−3(25 °C)[1] 1.3007 g·cm−3(30 °C)[2] |
| 熔点 | 18—21 °C(291—294 K)[1] |
| 沸点 | 233—236 °C(506—509 K)[1] 234.5 °C(507.6 K)[3] |
| 溶解性(水) | 0.043 g(25 °C)[4] |
| 危险性 | |
| 警示术语 | R:R22-R36/38[5] |
| 安全术语 | S:S26-S36/37[5] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
性质
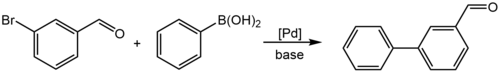
3-溴苯甲醛的C–Br键和醛基都可发生反应。例如,它可以和苯硼酸在钯催化剂和碱的存在下发生铃木偶联反应(C–C偶联),得到联苯-3-甲醛:[11][12]
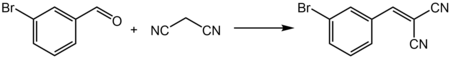
它和活泼亚甲基化合物可以发生克脑文盖尔缩合反应,例如和氰乙酸乙酯反应,生成3-(3-溴苯基)-2-氰基丙烯酸乙酯[13];和丙二腈反应,生成3-溴亚苄基丙二腈[14]:
它和胺可以发生C–N偶联反应,如和苄胺反应,得到N-(3-溴亚苄基)苄胺;[15]和4-氨基吗啉反应,得到N-(3-溴亚苄基)氨基吗啉。[16]
其醛基也可以发生氧化还原反应,如它可以被硼氢化钠还原为3-溴苯甲醇[17];在钯催化下被氢气还原为3-溴甲苯[18];或者被氧气[19]、过氧化叔丁醇[20]等氧化剂氧化为3-溴苯甲酸。
参考文献
- . Sigma-Aldrich. [2021-07-10].
- Bergmann, Ernst D.; Zimkin, E.; Pinchas, S. Reaction products of primary β-hydroxy amines with carbonyl compounds. II. Molecular refraction and infrared spectra. Recueil des Travaux Chimiques des Pays-Bas et de la Belgique, 1952. 71: 168-191. ISSN: 0370-7539. CODEN: RTCPB4.
- "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2021-07-10].
- Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 ((C) 1994-2021 ACD/Labs). Retrieved from SciFinder. [2021-07-10].
- . Alfa Aesar. [11 October 2013]. (原始内容存档于2015年6月10日).
- . ChemicalBook. [2021-05-10]. (原始内容存档于2021-05-10).
- 赵昊昱,吴朝华. 间溴苯甲醛. 医药中间体及其化工原料, 2004. 4: 31
- Lagerblom, Kalle; Wrigstedt, Pauli; Keskiväli, Juha; Parviainen, Arno; Repo, Timo. . ChemPlusChem. 2016, 81 (11): 1160–1165. ISSN 2192-6506. doi:10.1002/cplu.201600240.
- Wang, Lianyue; Bie, Zhixing; Shang, Sensen; Lv, Ying; Li, Guosong; Niu, Jingyang; Gao, Shuang. . RSC Advances. 2016, 6 (41): 35008–35013. ISSN 2046-2069. doi:10.1039/C6RA05536B.
- Balicki, Roman; Kaczmarek, Lukasz. . Synthetic Communications. 1991, 21 (17): 1777–1782. ISSN 0039-7911. doi:10.1080/00397919108021576.
- Li, Hongmiao; Tan, Xin; Zhang, Jianyong. . Chinese Journal of Chemistry. 2015, 33 (1): 141–146. ISSN 1001-604X. doi:10.1002/cjoc.201400479.
- Zawartka, Wojciech; Pośpiech, Piotr; Cypryk, Marek; Trzeciak, Anna M. . Journal of Molecular Catalysis A: Chemical. 2015, 407: 230–235. ISSN 1381-1169. doi:10.1016/j.molcata.2015.07.002.
- Jia, Yueqing; Fang, Yanjun; Zhang, Yingkui; Miras, Haralampos N.; Song, Yu-Fei. . Chemistry - A European Journal. 2015, 21 (42): 14862–14870. ISSN 0947-6539. doi:10.1002/chem.201501953.
- Zhao, Shen; Chen, Yang; Song, Yu-Fei. . Applied Catalysis A: General. 2014, 475: 140–146. ISSN 0926-860X. doi:10.1016/j.apcata.2014.01.017.
- Ou, Xiaoxu; Labes, Ricardo; Battilocchio, Claudio; Ley, Steven V. . Organic & Biomolecular Chemistry. 2018, 16 (36): 6652–6654. ISSN 1477-0520. doi:10.1039/C8OB01831F.
- Xu, Pan; Wang, Guoqiang; Zhu, Yuchen; Li, Weipeng; Cheng, Yixiang; Li, Shuhua; Zhu, Chengjian. . Angewandte Chemie International Edition. 2016, 55 (8): 2939–2943. ISSN 1433-7851. doi:10.1002/anie.201508698.
- Rayati, Saeed; Bohloulbandi, Elaheh; Zakavi, Saeed. . Inorganic Chemistry Communications. 2015, 54: 38–40. ISSN 1387-7003. doi:10.1016/j.inoche.2015.02.004.
- Wang, Shuguo; Zhou, Peng; Jiang, Liang; Zhang, Zehui; Deng, Kejian; Zhang, Yuhua; Zhao, Yanxi; Li, Jinlin; Bottle, Steven; Zhu, Huaiyong. . Journal of Catalysis. 2018, 368: 207–216. ISSN 0021-9517. doi:10.1016/j.jcat.2018.10.017.
- Mukhopadhyay, Chhanda; Datta, Arup. . Catalysis Communications. 2008, 9 (15): 2588–2592. ISSN 1566-7367. doi:10.1016/j.catcom.2008.07.019.
- Yang, Fan; Qiu, Tian; Chi, Cheng; Liang, Shuang; Deng, Lei; Wang, Xuyang; Wang, Chunxia; Fu, Jingyi; Wang, Ying; Li, Yongfeng. . Chemical Engineering Journal. 2017, 330: 880–889. ISSN 1385-8947. doi:10.1016/j.cej.2017.08.022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
-2-cyanoacrylate.png.webp)