 | |
| Names | |
|---|---|
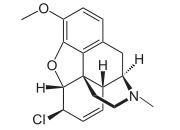
| IUPAC name
6β-Chloro-3-methoxy-17-methyl-7,8-didehydro-4,5α-epoxymorphinan | |
| Systematic IUPAC name
(4R,4aR,7R,7aR,12bS)-7-Chloro-9-methoxy-2,3,4,4a,7,7a-hexahydro-1H-4,12-methano[1]benzofuro[3,2-e]isoquinoline | |
| Other names
6β-Chloro-6-deoxycodeine 6-Chloro-6-deoxyisocodeine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H20ClNO2 | |
| Molar mass | 317.81 g·mol−1 |
| Melting point | 151 to 154 °C (304 to 309 °F; 424 to 427 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
α-Chlorocodide is an opioid analog that is a derivative of codeine in which the 6-hydroxy group has been replaced by chlorine.[1]
See also
References
- 1 2 Stork, Gilbert; Clarke, Frank H. (1956). "The SN2' Reaction. III. Structure and SN2' Reactions of the Halocodides". Journal of the American Chemical Society. 78 (18): 4619–4624. doi:10.1021/ja01599a026.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.