 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
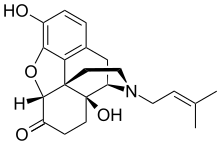
| Formula | C21H25NO4 |
| Molar mass | 355.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nalmexone (INN) (code names EN-1620A, UM-592), or nalmexone hydrochloride (USAN), is a semisynthetic, opioid partial agonist or mixed agonist-antagonist with both analgesic and narcotic antagonist properties that was never marketed.[1][2][3][4] In clinical studies it was found to have comparable analgesic efficacy to morphine, though with several-fold reduced potency.[5] In addition, nalmexone's side effects, the most common of which were sleepiness and sweating, were reported to be similar to those of morphine, albeit with a noticeably higher degree of incidence.[5]
Synthesis
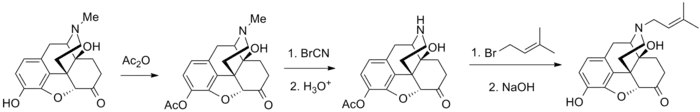
Nalmexone synthesis: Lowenstein, M. J.; Fishman, J.; 1967, U.S. Patent 3,320,262
See also
References
- ↑ Macdonald F (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1395. ISBN 978-0-412-46630-4. Retrieved 11 May 2012.
- ↑ Casy AF, Parfitt RT (1986). Opioid Analgesics: Chemistry and Receptors. Springer. p. 55. ISBN 978-0-306-42130-3. Retrieved 11 May 2012.
- ↑ Loew GH, Berkowitz DS (1978). "Quantum chemical studies of N-substituent variation in the oxymorphone series of opiate narcotics". Journal of Medicinal Chemistry. 21 (1): 101–106. doi:10.1021/jm00199a018. PMID 73588.
- ↑ Forrest WH, Shroff PF, Mahler DL (1972). "Analgesic and other effects of nalmexone in man". Clinical Pharmacology and Therapeutics. 13 (4): 520–525. doi:10.1002/cpt1972134520. PMID 4557582. S2CID 30780581.
- 1 2 Committee on Problems of Drug Dependence (1969). Bulletin, problems of drug dependence. National Academies. p. 5873. NAP:10503. Retrieved 11 May 2012.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.