 | |
| Names | |
|---|---|
| IUPAC name
3H-1-benzofuran-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.230 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6O2 | |
| Melting point | 49–51 °C[1] |
| Hazards | |
| GHS labelling:[2] | |
 | |
| Warning | |
| H315, H317, H319 | |
| P261, P264, P264+P265, P272, P280, P302+P352, P305+P351+P338, P321, P332+P317, P333+P317, P337+P317, P362+P364, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
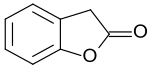
2-Cumaranone is a bicyclic heteroaromatic compound in which a six-membered benzene ring is annulated with a five-membered γ-butyrolactone ring. The 2(3H)-benzofuranone can also be considered as a lactone of (2-hydroxyphenyl)acetic acid. The benzofuranone basic structure is the basis of some natural products – such as rosmadial,[3] which is isolatable from rosemary oil, and some substances with high pharmacological activity, such as griseofulvin and rifampicin. Furthermore, 2-cumaranone is utilized as a starting material for the preparation of chemiluminescent and fluorescent dyes, for synthetic pharmaceutical agents, like the antiarrhythmic drug dronedarone, and especially for the fungicide azoxystrobin.
Occurrence and synthesis
In 1884, Adolf von Baeyer and Paul Fritsch disclosed the synthesis of 2-coumaranone, which they described as the lactone of o-oxyphenylacetic acid, through the distillation "over free fire" of (2-hydroxyphenyl)acetic acid.[4]
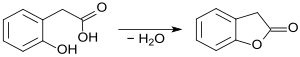
The lactone 3H-benzofuran-2-one forms in this process under intramolecular water splitting at high temperature and in an impure state.
A similar fragmentation by oxidative intramolecular ring closure from phenylacetic acid also yields only modest returns (< 20%) due to the oxidation sensitivity of the methylene group and the formation of several by-products 2-coumaranone.[5]
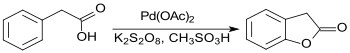
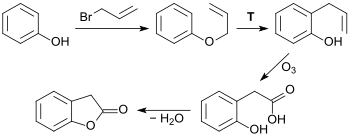
The Ozonolysis of 2-allylphenol obtained by the alkylation of phenol with 3-bromopropene to produce phenylallyl ether and subsequently its Claisen rearrangement, gives rise to 2-hydroxyphenylacetic acid, which, through water splitting, yields 2-coumaranone. Despite this method having good yields, its economic and safety considerations make it unsuitable for an industrial process.[6]
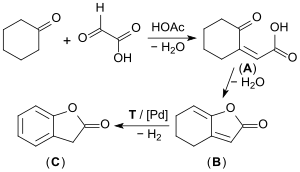
On an industrial scale, the well-filtered starting materials cyclohexanone and glyoxylic acid are first transformed in an acid-catalyzed aldol condensation to form the (predominantly) cis-2-oxocyclohexylidene acetic acid (A). This then, in a second step, is transformed into the so-called enollactone (B) through water elimination (90% yield). The enollactone is continuously dehydrogenated at 250 °C in the vapor phase on a palladium catalyst to form 2-coumaranone (C) dehydrogenation (yield approximately 67%).[7][8][9]
An alternative process that uses glyoxylic acid methyl ester methylhemiacetals rather than glyoxylic acid as a starting material has not gained widespread acceptance.[10]
Properties
Pure 2-coumaranone manifests as an off-white to pale yellow solid with an aromatic odor.[1] On purification by distillation, "a colorless oil passes which solidifies in the receiver into splendid, transparent, well-formed crystals".[11] 3H-benzofuran-2-one is soluble in hot water, diethyl ether[4] and acetonitrile.[10] The lactone hydrolyzes slowly in hot water and rapidly in aqueous alkalis to form 2-hydroxyphenylacetic acid or its alkali salt.[4]
Applications
5-Nitro-3H-benzofuran-2-one is formed during the nitration of 2-coumaranone with nitrating acid.[12][13]
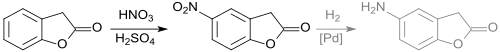
The 5-amino-3H-benzofuran-2-one can be obtained from the nitro compound using catalytic hydrogenation at a palladium catalyst.[12]
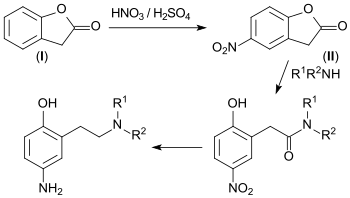
Lactones such as 2-coumaranone ('I) are readily cleaved by nucleophiles, leading to ring opening. Thus, 5-nitro-3H-benzofuran-2-one reacts with secondary amines to form 2-hydroxyphenylacetic acid amides. Through hydrogenation, these transform into corresponding 3-amino-6-hydroxyphenylethylamines, which are useful precursors for hair dyeing.[13]
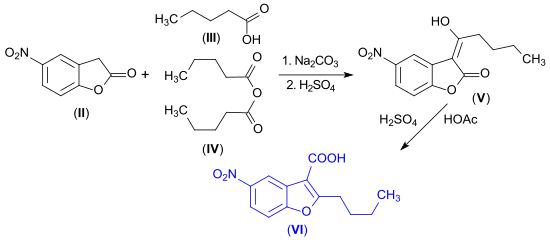
Condensation of 5-nitro-3H-benzofuran-2-one (II) with a mixture of valeric acid (III) and valeric anhydride (IV) results in the enollactone (V), which upon heating rearranges to the substituted benzofurancarboxylic acid (VI), a key precursor for the antiarrhythmic drug Dronedarone.[14]
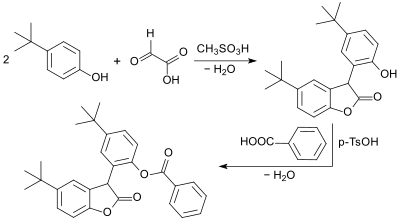
The basic structure of 2-coumaranone also underlies a class of antioxidants and radical scavengers, especially for stabilizing polypropylenes. In the synthesis of a model compound, glyoxylic acid reacts with 2 moles of 4-tert-butylphenol in the presence of methanesulfonic acid CH3SO3H to form a phenolic intermediate and is then esterified with benzoic acid.[15]
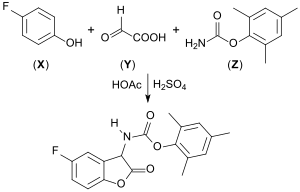
A one-pot reaction, carried out as a Tscherniak-Einhorn reaction of fluorophenols (X), glyoxylic acid (Y), and carbamates, such as carbamic acid methyl ester[16] or carbamic acid mesityl ester (Z), yields 2-coumaranones with carbamide side chains. These compounds react with strong bases such as diazabicycloundecene or potassium tert-butanolate, and show pronounced chemiluminescence in the presence of oxygen.[17][18][19]
The most notable application of 2-coumaranone by volume is as a starting material for the synthesis of the fungicide azoxystrobin[20] (known as Amistar from Syngenta) in the class of strobilurins.
References
- 1 2 Sigma-Aldrich Co., product no. {{{id}}}.
- ↑ "2-Coumaranone". pubchem.ncbi.nlm.nih.gov.
- ↑ N. Nakatani; R. Inatani (1983), "A New Diterpene Lactone, Rosmadial, from Rosemary (Rosmarinus officinalis L.)", Biosci. Biotechnol. Biochem., vol. 47, no. 2, pp. 353–358, doi:10.1080/00021369.1983.10865620
- 1 2 3 A. Baeyer; P. Fritsch (1884), "Ueber die o-Oxyphenylessigsäure und ihre Derivate", Chem. Ber., vol. 17, no. 1, pp. 973–975, doi:10.1002/cber.188401701258
- ↑ T. Fukagawa; Y. Fujiwara; H. Taniguchi (1982), "Palladium-catalyzed intramolecular aromatic nuclear acyloxylation: preparation of 2-coumaranone", J. Org. Chem., vol. 47, no. 12, pp. 2491–2493, doi:10.1021/jo00133a055
- ↑ EP 1481959, W. Jary, "Verfahren zur Herstellung von Lactonen und von aromatischen Hydroxycarbonsäuren", published 2004-12-01, assigned to DSM Fine Chemicals Austria Nfg GmbH & CO., KG
- ↑ N. Carmona; P. Gallezot; A. Perrard; L. Carmona; G. Mattioda; J.-C. Vallejos (1998), Synthesis of 2-Coumaranone by Catalytic Dehydrogenation of α-Carboxymethylidene Cyclohexanone, in Catalysis of Organic Reactions, Frank E. Herkes, editor, New York, NY, U.S.A.: Marcel Dekker, Inc., pp. 381–390, ISBN 0-8247-1929-8
- ↑ US 5616733, J.-C. Vallejos; A. Perrard & Y. Christidis et al., "Preparation method for 2-coumaranone", published 1997-4-1, assigned to Société Française Hoechst
- ↑ EP 0818451, N. Carmona; L. Carmona & A. Perrard et al., "Procédé de préparation de l'énollactone de l'acide 2-oxocyclohexylidène acétique et application à la préparation de la 2-coumaranone", published 1998-01-14, assigned to Clariant Chimie S.A.
- 1 2 EP 149838, M. Stanek; P. Hildebrand & C. Zimmermann et al., "Verfahren zur Herstellung von 2-Coumaron und substituierten 2-Coumaronen", published 2004-08-25, assigned to DSM Fine Chemicals Austria Nfg GmbH & CO., KG
- ↑ S. Czaplicki; Stanislaus von Kostanecki; V. Lampe (1909), "Versuche zur Synthese des Chromenols und seiner Derivate", Chem. Ber., vol. 42, no. 1, pp. 827–838, doi:10.1002/cber.190904201133
- 1 2 Christopher E. Malmberg (2015). "Total Synthesis of Clavatadine A Analogs to Produce a Viable Reversible Inhibitor for Factor XIa" (PDF). MS Thesis. Central Washington University Central Washington University. p. 14. Retrieved 2022-06-20.
- 1 2 US 7070630, M.-I. Lim, Y.-G. Pan, "Primary intermediates für oxidative coloration of hair", published 2006-4-7, assigned to The Procter & Gamble Co.
- ↑ EP 2508517, A. Shoutteeten, F. Bleger, F. Mordacq, J. Piron, "Process for the preparation of N-alkyl-2(hydroxy-4-benzoyl)-3-benzofurans and its intermediates thereof", published 2012-10-10, assigned to Clariant Specialty Fine Chemicals (France)
- ↑ EP 2500341, C.-F. Chiu, C.-Y. Su, S. Lee, "Benzofuranone derivatives and application of the same", published 2013-06-26, assigned to Chitec Technology Co., Ltd., Double Bond Chemical Ind., Co., Ltd., FDC, Lees Chemical Industry Co. Ltd.
- ↑ R. Krieg; B. Hoffmann; D. Weiß; C. Biskup (2019), "First synthesis of highly chemiluminescent benzo[b]furan-2(3H)-ones bearing a urea substructure", Helv. Chim. Acta, 102 (6): e1800243, doi:10.1002/hlca.201800243, S2CID 107893512
- ↑ S. Schramm; et al. (2013), "Investigations on the synthesis and chemiluminescence of novel 2-coumaranones", Arkivoc, vol. 3, pp. 174–188
- ↑ S. Schramm; et al. (2015), "Investigations on the synthesis and chemiluminescence of novel 2-coumaranones – II", Arkivoc, vol. 5, pp. 44–59
- ↑ "2-Coumaranone-1-L" (PDF; 170 kB). caymanchem.com. Cayman Chemical Co. Retrieved 2022-06-20.
- ↑ WO 199208703, J.D. Jones, G.A. DeBoos, P. Wilkinson, B.G. Cox, J.M. Fielden, "Process for the preparation of pyrimidine compounds", published 1992-5-29, assigned to Imperial Chemical Industries PLC