 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Insufflated, Rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
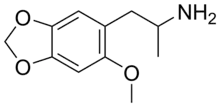
| Formula | C11H15NO3 |
| Molar mass | 209.245 g·mol−1 |
| 3D model (JSmol) | |
| |
MMDA-2 (2-methoxy-4,5-methylenedioxyamphetamine) is a psychedelic drug of the amphetamine class.[1] It is closely related to MMDA and MDA.[1]
Alexander Shulgin was likely the first to synthesize MMDA-2.[1] In his book PiHKAL, the dose is listed as 25–50 mg, and the duration is listed as 8–12 hours.[1] Shulgin reports that MMDA-2 produces effects such as enhanced awareness, empathy, and visual facilitation and distortion, as well as some side effects like gastrointestinal upset and appetite loss.[1] He states that 30 mg is very similar to 80 mg of MDA, and also remarks that it would be impossible for anyone to have a bad experience on the drug at that dose.[1]
Scientific research has shown that MMDA-2, unlike MMDA, but similarly to 6-methyl-MDA, is only very weak at inducing the release of serotonin or dopamine,[2] and accordingly, does not produce amphetamine-like responses in animals in drug discrimination studies.[3] Instead, MMDA-2 is likely to act as a pure 5-HT2 receptor agonist similarly to the DOx series of compounds, with activation of the 5-HT2A receptor conferring its psychedelic effects.[4]
MMDA-2 has been sold as a designer drug in Japan.[5]
See also
References
- 1 2 3 4 5 6 Shulgin A, Shulgin A (13 May 2016). "MMDA-2 (2-Methoxy-4,5-methylenedioxyamphetamine)". Pihkal: A Chemical Love Story. Transform Press. ISBN 978-0-9630096-0-9.
- ↑ McKenna DJ, Guan XM, Shulgin AT (March 1991). "3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine". Pharmacology, Biochemistry, and Behavior. 38 (3): 505–512. doi:10.1016/0091-3057(91)90005-M. PMID 1829838. S2CID 2740262.
- ↑ Glennon RA, Yousif M, Naiman N, Kalix P (March 1987). "Methcathinone: a new and potent amphetamine-like agent". Pharmacology, Biochemistry, and Behavior. 26 (3): 547–551. doi:10.1016/0091-3057(87)90164-X. PMID 3575369. S2CID 5890314.
- ↑ Clare BW (2002). "QSAR of benzene derivatives: comparison of classical descriptors, quantum theoretic parameters and flip regression, exemplified by phenylalkylamine hallucinogens". Journal of Computer-Aided Molecular Design. 16 (8–9): 611–633. Bibcode:2002JCAMD..16..611C. doi:10.1023/A:1021966231380. PMID 12602954. S2CID 9948738.
- ↑ Min JZ, Shimizu Y, Toyo'oka T, Inagaki S, Kikura-Hanajiri R, Goda Y (October 2008). "Simultaneous determination of 11 designated hallucinogenic phenethylamines by ultra-fast liquid chromatography with fluorescence detection". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 873 (2): 187–194. doi:10.1016/j.jchromb.2008.08.020. PMID 18789774.