 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Insufflation, Rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
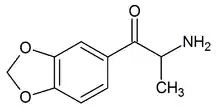
| Formula | C10H11NO3 |
| Molar mass | 193.202 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
3,4-Methylenedioxycathinone (also known as MDC, Nitrilone, Amylone and βk-MDA) is an empathogen and stimulant of the phenethylamine, amphetamine, and cathinone classes[1][2] and the β-keto analogue of MDA.[3]
Methylenedioxycathinone has been investigated as antidepressant and antiparkinson agent.[4]
See also
References
- ↑ Zaitsu K, Katagi M, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K (July 2011). "Recently abused β-keto derivatives of 3,4-methylenedioxyphenylalkylamines: a review of their metabolisms and toxicological analysis". Forensic Toxicology. 29 (2): 73–84. doi:10.1007/s11419-011-0111-8. S2CID 25416491.
- ↑ Dal Cason TA, Young R, Glennon RA (December 1997). "Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs". Pharmacology, Biochemistry, and Behavior. 58 (4): 1109–16. doi:10.1016/S0091-3057(97)00323-7. PMID 9408221. S2CID 9704972.
- ↑ Dal Cason TA (May 1997). "The characterization of some 3,4-methylenedioxycathinone (MDCATH) homologs". Forensic Science International. 87 (1): 9–53. doi:10.1016/S0379-0738(97)02133-6.
- ↑ WO 1996039133, Jacob III P, Shulgin AT, "Preparation of novel N-substituted-2-amino-3′,4′-methylenedioxypropiophenones as anti-depressant and anti-parkinsonism agents.", published 12 December 1996, assigned to Neurobiological Technologies, Inc.
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.