 | |
| Names | |
|---|---|
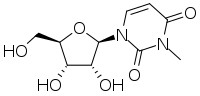
| IUPAC name
3-Methyluridine | |
| Systematic IUPAC name
1-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3-methylpyrimidine-2,4(1H,3H)-dione | |
| Other names
N3-Methyluridine; N-3-Methyluridine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H14N2O6 | |
| Molar mass | 258.230 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The chemical compound 3-methyluridine, also called N3-methyluridine, is a pyrimidine nucleoside (abbreviated m3U). In living organisms it is present as RNA modification which has been detected in 23S rRNA of archaea, 16S and 23S rRNA of eubacteria, and 18S, 25S, and 28S of eukaryotic ribosomal RNAs.[1]
See also
References
- ↑ Desaulniers, Jean-Paul; Chui, Helen M.-P.; Chow, Christine S. (2005-12-15). "Solution conformations of two naturally occurring RNA nucleosides: 3-methyluridine and 3-methylpseudouridine". Bioorganic & Medicinal Chemistry. 13 (24): 6777–6781. doi:10.1016/j.bmc.2005.07.061. ISSN 0968-0896. PMID 16125393.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.