 | |
| Names | |
|---|---|
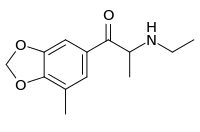
| Preferred IUPAC name
2-(Ethylamino)-1-(7-methyl-2H-1,3-benzodioxol-5-yl)ethan-1-one | |
| Other names
R-MMC; 3,4-Methylenedioxy-5-methyl-N-ethyl cathinone; 5-Methyl-βk-MDEA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C13H17NO3 | |
| Molar mass | 235.283 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5-Methylethylone (5-methyl-βk-MDEA, 5ME) is an empathogen, stimulant and psychedelic drug of the amphetamine, phenethylamine, and cathinone chemical classes.[1] It is structurally related to ethylone, a novel designer drug. Relatively little data exists about the pharmacological properties, metabolism, and toxicity of 5-methylethylone, though it has been sold as a designer drug.[2][3]
Legal status
United States
5-Methylethylone is unscheduled in the United States, but it is not currently approved by the Food and Drug Administration for human consumption. The state of Vermont lists it as a regulated drug.[4]
See also
References
- ↑ "3,4-Methylenedioxy-5-methylethcathinone (hydrochloride)]". Cayman Chemical.
- ↑ Assi S, Gulyamova N, Kneller P, Osselton D (May 2017). "The effects and toxicity of cathinones from the users' perspectives: A qualitative study". Human Psychopharmacology. 32 (3): e2610. doi:10.1002/hup.2610. PMID 28631397.
- ↑ Schifano F, Napoletano F, Arillotta D, Zangani C, Gilgar L, Guirguis A, et al. (March 2020). "The clinical challenges of synthetic cathinones". British Journal of Clinical Pharmacology. 86 (3): 410–419. doi:10.1111/bcp.14132. hdl:2299/21881. PMC 7080616. PMID 31674690.
- ↑ Regulated Drugs Rule, Vermont Health Regulations
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.