 | |
| Clinical data | |
|---|---|
| Other names | 5F-MDMB-2201; 5-Fluoro MDMB-PICA |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
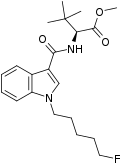
| Formula | C21H29FN2O3 |
| Molar mass | 376.472 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
5F-MDMB-PICA is a designer drug and synthetic cannabinoid.[2][3][4][5][6] In 2018, it was the fifth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.[7]
5F-MDMB-PICA is a potent agonist of both the CB1 receptor and the CB2 receptor with EC50 values of 0.45 nM and 7.4 nM, respectively.[8]
In the United States, 5F-MDMB-PICA was temporarily emergency scheduled by the DEA in 2019.[9] In December 2019, the UNODC announced scheduling recommendations placing 5F-MDMB-PICA into Schedule II.[10] In the United States 5F-MDMB-PICA was made a permanent Schedule I Controlled Substance nationwide on April 7, 2022.[11]
References
- ↑ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ↑ Risseeuw MD, Blanckaert P, Coopman V, Van Quekelberghe S, Van Calenbergh S, Cordonnier J (April 2017). "Identification of a new tert-leucinate class synthetic cannabinoid in powder and "spice-like" herbal incenses: Methyl 2-[[1-(5-fluoropentyl)indole-3-carbonyl]amino]-3,3-dimethyl-butanoate (5F-MDMB-PICA)". Forensic Science International. 273: 45–52. doi:10.1016/j.forsciint.2017.01.023. hdl:1854/LU-8542830. PMID 28214755.
- ↑ Mogler L, Franz F, Rentsch D, Angerer V, Weinfurtner G, Longworth M, et al. (January 2018). "Detection of the recently emerged synthetic cannabinoid 5F-MDMB-PICA in 'legal high' products and human urine samples". Drug Testing and Analysis. 10 (1): 196–205. doi:10.1002/dta.2201. PMID 28371476.
- ↑ Szpot P, Nowak K, Wachełko O, Tusiewicz K, Chłopaś-Konowałek A, Zawadzki M (January 2023). "Methyl (S)-2-(1-7 (5-fluoropentyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate (5F-MDMB-PICA) intoxication in a child with identification of two new metabolites (ultra-high-performance liquid chromatography-tandem mass spectrometry)". Forensic Toxicology. 41 (1): 47–58. doi:10.1007/s11419-022-00629-7. PMID 36652054. S2CID 249318941.
- ↑ Tokarczyk B, Jurczyk A, Krupińska J, Adamowicz P (December 2022). "Fatal intoxication with new synthetic cannabinoids 5F-MDMB-PICA and 4F-MDMB-BINACA-parent compounds and metabolite identification in blood, urine and cerebrospinal fluid". Forensic Science, Medicine, and Pathology. 18 (4): 393–402. doi:10.1007/s12024-022-00492-3. PMC 9194349. PMID 35699867.
- ↑ Wagmann L, Stiller RG, Fischmann S, Westphal F, Meyer MR (October 2022). "Going deeper into the toxicokinetics of synthetic cannabinoids: in vitro contribution of human carboxylesterases". Archives of Toxicology. 96 (10): 2755–2766. doi:10.1007/s00204-022-03332-z. PMC 9352624. PMID 35788413.
- ↑ "Emerging Threat Report: Annual 2018" (PDF). Special Testing and Research Laboratory, Drug Enforcement Administration. Archived from the original (PDF) on 2019-08-01. Retrieved 2019-08-02.
- ↑ Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, et al. (September 2016). "Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues". ACS Chemical Neuroscience. 7 (9): 1241–1254. doi:10.1021/acschemneuro.6b00137. PMID 27421060.
- ↑ "Schedules of Controlled Substances: Temporary Placement of 5F-EDMB-PINACA, 5F-MDMB-PICA, FUB-AKB48, 5F-CUMYL-PINACA, and FUB-144 into Schedule I". Federal Register. 2019-04-16.
- ↑ "December 2019 – WHO: World Health Organization recommends 12 NPS for scheduling".
- ↑ "Schedules of Controlled Substances: Placement of 5F-EDMB-PINACA, 5FMDMB-PICA, FUB-AKB48, 5F-CUMYLPINACA, and FUB-144 in Schedule I" (PDF). Federal Register. 87 (67). 2022-04-07.
Further reading
- Musa A, Simola N, Piras G, Caria F, Onaivi ES, De Luca MA (December 2020). "Neurochemical and Behavioral Characterization after Acute and Repeated Exposure to Novel Synthetic Cannabinoid Agonist 5-MDMB-PICA". Brain Sciences. 10 (12): 1011. doi:10.3390/brainsci10121011. PMC 7766979. PMID 33353194.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.