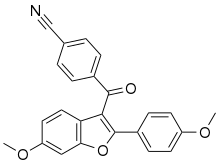
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.190.302 |
| Chemical and physical data | |
| Formula | C24H17NO4 |
| Molar mass | 383.395 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
LY-320,135 is a drug used in scientific research which acts as a selective antagonist of the cannabinoid receptor CB1. It was developed by Eli Lilly and Company in the 1990s.
LY-320,135 displays fairly good selectivity, with a binding affinity for CB1 around 70x stronger than for CB2,[1] but both its potency and selectivity are modest compared to newer agents, and at higher doses it also binds to a range of non-cannabinoid receptors. However LY-320,135 is still fairly widely used in research, particularly for elucidating the mechanisms by which many CB1 antagonists act as inverse agonists at higher doses.[2]
References
- ↑ Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, et al. (January 1998). "LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation". The Journal of Pharmacology and Experimental Therapeutics. 284 (1): 291–7. PMID 9435190.
- ↑ Pertwee RG (February 2005). "Inverse agonism and neutral antagonism at cannabinoid CB1 receptors". Life Sciences. 76 (12): 1307–24. doi:10.1016/j.lfs.2004.10.025. PMID 15670612.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.