 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.017.146 |
| Chemical and physical data | |
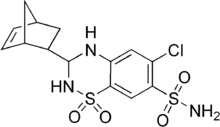
| Formula | C14H16ClN3O4S2 |
| Molar mass | 389.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cyclothiazide (Anhydron, Acquirel, Doburil, Fluidil, Renazide, Tensodiural, Valmiran), sometimes abbreviated CTZ, is a benzothiadiazide (thiazide) diuretic and antihypertensive that was originally introduced in the United States in 1963 by Eli Lilly and was subsequently also marketed in Europe and Japan.[1][2] Related drugs include diazoxide, hydrochlorothiazide, and chlorothiazide.[3]
In 1993, it was discovered that cyclothiazide is a positive allosteric modulator of the AMPA and kainate receptors, capable of reducing or essentially eliminating rapid desensitization of the former receptor, and potentiating AMPA-mediated glutamate currents by as much as 18-fold at the highest concentration tested (100 μM).[3][4][5][6] Additionally, in 2003, cyclothiazide was also found to act as a GABAA receptor negative allosteric modulator, potently inhibiting GABAA-mediated currents.[7] In animals it is a powerful convulsant, robustly enhancing epileptiform activity and inducing seizures, but without producing any apparent neuronal death.[8][9]
Cyclothiazide has been found to act as a non-competitive antagonist of the mGluR1.[10] It is selective for mGluR1 over other metabotropic glutamate receptors.[10]
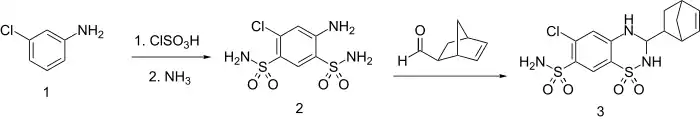
Synthesis
See also
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. p. 1932. ISBN 978-3-88763-075-1.
- ↑ Sittig M (1988). Pharmaceutical manufacturing encyclopedia. Park Ridge, N.J., U.S.A: Noyes Publications. p. 1756. ISBN 978-0-8155-1144-1.
- 1 2 Skolnick P, Palfreyman MG, Reynolds IJ (1994). Direct and allosteric control of glutamate receptors. Boca Raton: CRC Press. p. 174. ISBN 978-0-8493-8307-6.
- ↑ Yamada KA, Tang CM (September 1993). "Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents". The Journal of Neuroscience. 13 (9): 3904–3915. doi:10.1523/JNEUROSCI.13-09-03904.1993. PMC 6576449. PMID 8103555.
- ↑ Bertolino M, Baraldi M, Parenti C, Braghiroli D, DiBella M, Vicini S, Costa E (1993). "Modulation of AMPA/kainate receptors by analogues of diazoxide and cyclothiazide in thin slices of rat hippocampus". Receptors & Channels. 1 (4): 267–278. PMID 7915948.
- ↑ Parsons CG, Danysz W, Zieglgänsberger W (2005). "Excitatory Amino Acid Neurotransmission". In Ströhle A, Bilkei-Gorzo A, Holsboer F (eds.). Anxiety and anxiolytic drugs. Berlin: Springer. p. 566. ISBN 978-3-540-22568-3.
- ↑ Deng L, Chen G (October 2003). "Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses". Proceedings of the National Academy of Sciences of the United States of America. 100 (22): 13025–13029. Bibcode:2003PNAS..10013025D. doi:10.1073/pnas.2133370100. PMC 240738. PMID 14534329.
- ↑ Qi J, Wang Y, Jiang M, Warren P, Chen G (March 2006). "Cyclothiazide induces robust epileptiform activity in rat hippocampal neurons both in vitro and in vivo". The Journal of Physiology. 571 (Pt 3): 605–618. doi:10.1113/jphysiol.2005.103812. PMC 1805799. PMID 16423850.
- ↑ Kong S, Qian B, Liu J, Fan M, Chen G, Wang Y (October 2010). "Cyclothiazide induces seizure behavior in freely moving rats". Brain Research. 1355: 207–213. doi:10.1016/j.brainres.2010.07.088. PMC 2947190. PMID 20678492.
- 1 2 Surin A, Pshenichkin S, Grajkowska E, Surina E, Wroblewski JT (March 2007). "Cyclothiazide selectively inhibits mGluR1 receptors interacting with a common allosteric site for non-competitive antagonists". Neuropharmacology. 52 (3): 744–754. doi:10.1016/j.neuropharm.2006.09.018. PMC 1876747. PMID 17095021.
- ↑ Whitehead CW, Traverso JJ, Sullivan HR, Marshall FJ (1961). "Diuretics. V. 3,4-Dihydro-1,2,4-benzothiadiazine 1,1-Dioxides". The Journal of Organic Chemistry. 26 (8): 2814–2818. doi:10.1021/jo01066a046.
- ↑ US 3275625, Müller E, Hasspacher K, issued 1966, assigned to Boehringer Ingelheim