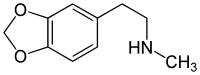
 | |
| Clinical data | |
|---|---|
| Other names | 1,3-benzodioxolyl-N-methyl-5-ethanamine; 3,4-methylenedioxy-N-methyl-2-phenylethylamine; Norlobivine |
| Routes of administration | Various |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Homarylamine (INN;[1] also known as 3,4-methylenedioxy-N-methylphenethylamine and MDMPEA) is an antitussive (anti-cough) drug[2] which was patented in 1956 by Merck & Co.,[3] but has never been used medically as such.
Chemically it is a substituted phenethylamine. It is the N-methylated analog of methylenedioxyphenethylamine (MDPEA). It is a schedule I drug in the USA as a positional isomer of MDA.
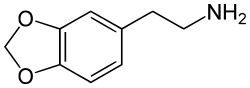
Methylenedioxyphenethylamine (MDPEA) for comparison
Reactions
Reaction of homoarylamine with formaldehyde gives hydrastinine.
See also
- Hydrastine, an alkaloid derivative of homarylamine
References
- ↑ "International Non-Proprietary Names for Pharmaceutical Preparations" (PDF). Chronicle of the World Health Organization. 12 (3). 1958.
- ↑ Stefko PL, Denzel J, Hickey I (March 1961). "Experimental Investigation of Nine Antitussive Drugs". Journal of Pharmaceutical Sciences. 50 (3): 216–221. doi:10.1002/jps.2600500309.
- ↑ U.S. Patent 2,820,739
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.