| Phthiraptera Temporal range: | |
|---|---|
 | |
| Light micrograph of Fahrenholzia pinnata | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Psocodea |
| Suborder: | Troctomorpha |
| Infraorder: | Nanopsocetae |
| Parvorder: | Phthiraptera Haeckel, 1896 |
| Clades[1] | |
Louse (pl.: lice) is the common name for any member of the clade Phthiraptera, which contains nearly 5,000 species of wingless parasitic insects. Phthiraptera has variously been recognized as an order, infraorder, or a parvorder, as a result of developments in phylogenetic research.[1][2][3]
Lice are obligate parasites, living externally on warm-blooded hosts which include every species of bird and mammal, except for monotremes, pangolins, and bats. Lice are vectors of diseases such as typhus.
Chewing lice live among the hairs or feathers of their host and feed on skin and debris, whereas sucking lice pierce the host's skin and feed on blood and other secretions. They usually spend their whole life on a single host, cementing their eggs, called nits, to hairs or feathers. The eggs hatch into nymphs, which moult three times before becoming fully grown, a process that takes about four weeks. Genetic evidence indicates that lice are a highly modified lineage of Psocoptera (now called Psocodea), commonly known as booklice, barklice or barkflies. The oldest known fossil lice are from the Paleogene, though molecular clock estimates suggest that they originated earlier, during the Cretaceous.
Humans host two species of louse—the head louse and the body louse are subspecies of Pediculus humanus; and the pubic louse, Pthirus pubis. The body louse has the smallest genome of any known insect; it has been used as a model organism and has been the subject of much research. Lice were ubiquitous in human society until at least the Middle Ages. They appear in folktales, songs such as The Kilkenny Louse House, and novels such as James Joyce's Finnegans Wake. They commonly feature in the psychiatric disorder delusional parasitosis. A louse was one of the early subjects of microscopy, appearing in Robert Hooke's 1667 book, Micrographia.
Morphology and diversity
Lice are divided into two groups: sucking lice, which obtain their nourishment from feeding on the sebaceous secretions and body fluids of their host; and chewing lice, which are scavengers, feeding on skin, fragments of feathers or hair, and debris found on the host's body. Many lice are specific to a single species of host and have co-evolved with it. In some cases, they live on only a particular part of the body. Some animals are known to host up to fifteen different species, although one to three is typical for mammals, and two to six for birds. Lice generally cannot survive for long if removed from their host.[4] If their host dies, lice can opportunistically use phoresis to hitch a ride on a fly and attempt to find a new host.[5]
Sucking lice range in length from 0.5 to 5 mm (1⁄64 to 13⁄64 in). They have narrow heads and oval, flattened bodies. They have no ocelli, and their compound eyes are reduced in size or absent. Their antennae are short with three to five segments, and their mouthparts, which are retractable into their head, are adapted for piercing and sucking.[6] There is a cibarial pump at the start of the gut; it is powered by muscles attached to the inside of the cuticle of the head. The mouthparts consist of a proboscis which is toothed, and a set of stylets arranged in a cylinder inside the proboscis, containing a salivary canal (ventrally) and a food canal (dorsally).[7] The thoracic segments are fused, the abdominal segments are separate, and there is a single large claw at the tip of each of the six legs.[6]
Chewing lice are also flattened and can be slightly larger than sucking lice, ranging in length from 0.5 to 6 mm (1⁄64 to 15⁄64 in). They are similar to sucking lice in form but the head is wider than the thorax and all species have compound eyes. There are no ocelli and the mouthparts are adapted for chewing. The antennae have three to five segments and are slender in the suborder Ischnocera, but club-shaped in the suborder Amblycera. The legs are short and robust, and terminated by one or two claws. Some species of chewing lice house symbiotic bacteria in bacteriocytes in their bodies. These may assist in digestion because if the insect is deprived of them, it will die. Lice are usually cryptically coloured to match the fur or feathers of the host.[6][8] A louse's color varies from pale beige to dark gray; however, if feeding on blood, it may become considerably darker.
Female lice are usually more common than males, and some species are parthenogenetic, with young developing from unfertilized eggs. A louse's egg is commonly called a nit. Many lice attach their eggs to their hosts' hair with specialized saliva; the saliva/hair bond is very difficult to sever without specialized products. Lice inhabiting birds, however, may simply leave their eggs in parts of the body inaccessible to preening, such as the interior of feather shafts. Living louse eggs tend to be pale whitish, whereas dead louse eggs are yellower.[4] Lice are exopterygotes, being born as miniature versions of the adult, known as nymphs. The young moult three times before reaching the final adult form, usually within a month after hatching.[4]
Humans host three different kinds of lice: head lice, body lice, and pubic lice. Head lice and body lice are subspecies of Pediculus humanus, and pubic lice are a separate species, Pthirus pubis. Lice infestations can be controlled with lice combs, and medicated shampoos or washes.[9]
Ecology
The average number of lice per host tends to be higher in large-bodied bird species than in small ones.[10] Lice have an aggregated distribution across bird individuals, i.e. most lice live on a few birds, while most birds are relatively free of lice. This pattern is more pronounced in territorial than in colonial—more social—bird species.[11] Host organisms that dive under water to feed on aquatic prey harbor fewer taxa of lice.[12][13] Bird taxa that are capable of exerting stronger antiparasitic defense—such as stronger T cell immune response or larger uropygial glands—harbor more taxa of Amblyceran lice than others.[14][15] Reductions in the size of host populations may cause a long-lasting reduction of louse taxonomic richness,[16] for example, birds introduced into New Zealand host fewer species of lice there than in Europe.[17][18] Louse sex ratios are more balanced in more social hosts and more female-biased in less social hosts, presumably due to the stronger isolation among louse subpopulations (living on separate birds) in the latter case.[19] The extinction of a species results in the extinction of its host-specific lice. Host-switching is a random event that would seem very rarely likely to be successful, but speciation has occurred over evolutionary time-scales so it must be successfully accomplished sometimes.[16]
Lice may reduce host life expectancy if the infestation is heavy,[20] but most seem to have little effect on their host. The habit of dust bathing in domestic hens is probably an attempt by the birds to rid themselves of lice.[6] Lice may transmit microbial diseases and helminth parasites,[21] but most individuals spend their whole life cycle on a single host and are only able to transfer to a new host opportunistically.[6] Ischnoceran lice may reduce the thermoregulation effect of the plumage; thus heavily infested birds lose more heat than others.[22] Lice infestation is a disadvantage in the context of sexual rivalry.[23][24]
Evolution
Phthiraptera lice are members of Psocodea (formerly Psocoptera), the order that contains booklice, barklice and barkflies. Within Psocodea, lice are within the suborder Troctomorpha, and most closely related to the family Liposcelididae.[25] The oldest confirmed fossil louse is a bird louse, Megamenopon rasnitsyni, from Eckfelder Maar, Germany, which dates to the Eocene, around 44 million years ago.[26] Saurodectes vrsanskyi from the Early Cretaceous (Aptian) Zaza Formation of Buryatia, Russia, has also been suggested to be a louse, but this is tentative.[27]
Cladogram showing the position of Phthiraptera within Psocodea:[1]
| Psocodea |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Classification
Phthiraptera is clearly a monophyletic grouping, united as the members are by a number of derived features including their parasitism on warm-blooded vertebrates and the combination of their metathoracic ganglia with their abdominal ganglia to form a single ventral nerve junction.[28] The order has traditionally been divided into two suborders, the sucking lice (Anoplura) and the chewing lice (Mallophaga); however, subsequent classifications suggest that the Mallophaga are paraphyletic and four suborders were then recognized:[29]
- Anoplura: sucking lice, occurring on mammals exclusively
- Rhynchophthirina: parasites of elephants and warthogs
- Ischnocera: mostly avian chewing lice, with one family parasitizing mammals
- Amblycera: a primitive suborder of chewing lice, widespread on birds, and also occurring on South American and Australian mammals
Upon finding that Phthiraptera was nested within Psocoptera, Phthiraptera, in 2021 de Moya et al. proposed reducing the rank of Phthiraptera to infraorder, and the four suborders to parvorder.[1] These changes were accepted by Psocodea Species File and others, with the exception of placing Phthiraptera under the infraorder Nanopsocetae, as a parvorder, with the four subgroups listed above. These classifications are likely to change in the future as a result of ongoing phylogenetic research.[2][3]
Nearly 5,000 species of louse have been identified, about 4,000 being parasitic on birds and 800 on mammals. Lice are present on every continent in all the habitats that their host animals occupy.[29] They are found even in the Antarctic, where penguins carry 15 species of lice (in the genera Austrogonoides and Nesiotinus).[30] The oldest known record of the group is Megamenopon rasnitsyni from the Eocene of Germany, but it is essentially a modern form, belonging to Amblycera, so the group as a whole likely has an origin in the Mesozoic.[26]
.JPG.webp) Ricinus bombycillae, an amblyceran louse from a Bohemian waxwing
Ricinus bombycillae, an amblyceran louse from a Bohemian waxwing.JPG.webp) Trinoton anserinum, an amblyceran louse from a mute swan
Trinoton anserinum, an amblyceran louse from a mute swan Bovicola limbata, an ischnoceran louse from goats. The species is sexually dimorphic, with the male smaller than the female.
Bovicola limbata, an ischnoceran louse from goats. The species is sexually dimorphic, with the male smaller than the female.
Phylogeny
Lice have been the subject of significant DNA research in the 2000s that led to discoveries on human evolution. The three species of sucking lice that parasitize human beings belong to two genera, Pediculus and Pthirus: head lice (Pediculus humanus capitis), body lice (Pediculus humanus humanus), and pubic lice (Pthirus pubis). Human head and body lice (genus Pediculus) share a common ancestor with chimpanzee lice, while pubic lice (genus Pthirus) share a common ancestor with gorilla lice. Using phylogenetic and cophylogenetic analysis, Reed et al. hypothesized that Pediculus and Pthirus are sister taxa and monophyletic.[31] In other words, the two genera descended from the same common ancestor. The age of divergence between Pediculus and its common ancestor is estimated to be 6-7 million years ago, which matches the age predicted by chimpanzee-hominid divergence.[31] Because parasites rely on their hosts, host–parasite cospeciation events are likely.
Genetic evidence suggests that human ancestors acquired pubic lice from gorillas approximately 3-4 million years ago.[31] Unlike the genus Pediculus, the divergence in Pthirus does not match the age of host divergence that likely occurred 7 million years ago. Reed et al. propose a Pthirus species host-switch around 3-4 million years ago. While it is difficult to determine if a parasite–host switch occurred in evolutionary history, this explanation is the most parsimonious (containing the fewest evolutionary changes).[31]
Additionally, the DNA differences between head lice and body lice provide corroborating evidence that humans used clothing between 80,000 and 170,000 years ago, before leaving Africa.[32] Human head and body lice occupy distinct ecological zones: head lice live and feed on the scalp, while body lice live on clothing and feed on the body. Because body lice require clothing to survive, the divergence of head and body lice from their common ancestor provides an estimate of the date of introduction of clothing in human evolutionary history.[32][33]
The mitochondrial genome of the human species of the body louse (Pediculus humanus humanus), the head louse (Pediculus humanus capitis) and the pubic louse (Pthirus pubis) fragmented into a number of minichromosomes, at least seven million years ago.[34] Analysis of mitochondrial DNA in human body and hair lice reveals that greater genetic diversity existed in African than in non-African lice.[33][35] Human lice can also shed light on human migratory patterns in prehistory. The dominating theory of anthropologists regarding human migration is the Out of Africa Hypothesis. Genetic diversity accumulates over time, and mutations occur at a relatively constant rate. Because there is more genetic diversity in African lice, the lice and their human hosts must have existed in Africa before anywhere else.[35]
In human culture
In social history
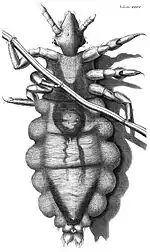
Lice have been intimately associated with human society throughout history. In the Middle Ages, they were essentially ubiquitous. At the death of Thomas Becket, Archbishop of Canterbury in 1170, it was recorded that "The vermin boiled over like water in a simmering cauldron, and the onlookers burst into alternate weeping and laughing".[36] The clergy often saw lice and other parasites as a constant reminder of human frailty and weakness. Monks and nuns would purposely ignore grooming themselves and suffer from infestations to express their religious devotion.[37] A mediaeval treatment for lice was an ointment made from pork grease, incense, lead, and aloe.[38]
Robert Hooke's 1667 book, Micrographia: or some physiological descriptions of minute bodies made by magnifying glasses with observations and Inquiries thereupon, illustrated a human louse, drawn as seen down an early microscope.[39]
Margaret Cavendish's satirical The Description of a New World, Called The Blazing-World (1668) has "Lice-men" as "mathematicians", investigating nature by trying to weigh the air like the real scientist Robert Boyle.[40][41]
In 1935 the Harvard medical researcher Hans Zinsser wrote the book Rats, Lice and History, alleging that both body and head lice transmit typhus between humans.[42] Despite this, the modern view is that only the body louse can transmit the disease.[43]

Soldiers in the trenches of the First World War suffered severely from lice, and the typhus they carried. The Germans boasted that they had lice under effective control, but themselves suffered badly from lice in the Second World War on the Eastern Front, especially in the Battle of Stalingrad. "Delousing" became a euphemism for the extermination of Jews in concentration camps such as Auschwitz under the Nazi regime.[44]
In the psychiatric disorder delusional parasitosis, patients express a persistent irrational fear of animals such as lice and mites, imagining that they are continually infested and complaining of itching, with "an unshakable false belief that live organisms are present in the skin".[45]
In science
The human body louse Pediculus humanus humanus has (2010) the smallest insect genome known.[46] This louse can transmit certain diseases while the human head louse (P. h. capitis), to which it is closely related, cannot. With their simple life history and small genomes, the pair make ideal model organisms to study the molecular mechanisms behind the transmission of pathogens and vector competence.[47]
In literature and folklore

James Joyce's 1939 book Finnegans Wake has the character Shem the Penman infested with "foxtrotting fleas, the lieabed lice, ... bats in his belfry".[50]
Clifford E. Trafzer's A Chemehuevi Song: The Resilience of a Southern Paiute Tribe retells the story of Sinawavi (Coyote)'s love for Poowavi (Louse). Her eggs are sealed in a basket woven by her mother, who gives it to Coyote, instructing him not to open it before he reaches home. Hearing voices coming from it, however, Coyote opens the basket and the people, the world's first human beings, pour out of it in all directions.[51]
The Irish songwriter John Lyons (b. 1934) wrote the popular[52] song The Kilkenny Louse House. The song contains the lines "Well we went up the stairs and we put out the light, Sure in less than five minutes, I had to show fight. For the fleas and the bugs they collected to march, And over me stomach they formed a great arch". It has been recorded by Christie Purcell (1952), Mary Delaney on From Puck to Appleby (2003), and the Dubliners on Double Dubliners (1972) among others.[52][53]
Robert Burns dedicated a poem to the louse, inspired by witnessing one on a lady's bonnet in church: "Ye ugly, creepin, blastid wonner, Detested, shunn'd, by saint and sinner, How dare ye set your fit upon her, sae fine lady! Gae somewhere else, and seek your dinner on some poor body." John Milton in Paradise Lost mentioned the biblical plague of lice visited upon pharaoh: "Frogs, lice, and flies must all his palace fill with loathed intrusion, and filled all the land." John Ray recorded a Scottish proverb, "Gie a beggar a bed and he'll repay you with a Louse." In Shakespeare's Troilus and Cressida, Thersites compares Menelaus, brother of Agamemnon, to a louse: "Ask me not what I would be, if I were not Thersites; for I care not to be the louse of a lazar, so I were not Menelaus."[54]
Woodlouse
The name woodlouse or wood-louse is given to crustaceans of the suborder Oniscidea within the order Isopoda, unrelated to lice. The Oxford English Dictionary's earliest citation of this usage is from 1611.[55]
See also
References
- 1 2 3 4 de Moya, Robert S; Yoshizawa, Kazunori; Walden, Kimberly K O; Sweet, Andrew D; Dietrich, Christopher H; Kevin P, Johnson (2021-06-16). Buckley, Thomas (ed.). "Phylogenomics of Parasitic and Nonparasitic Lice (Insecta: Psocodea): Combining Sequence Data and Exploring Compositional Bias Solutions in Next Generation Data Sets". Systematic Biology. 70 (4): 719–738. doi:10.1093/sysbio/syaa075. ISSN 1063-5157. PMID 32979270.
- 1 2 Johnson, Kevin P.; Smith, Vincent S. (2021). "Psocodea species file online, Version 5.0". Retrieved 2021-11-03.
- 1 2 "Catalogue of Life, Phthiraptera Haeckel, 1896". 2021. Retrieved 2021-11-03.
- 1 2 3 Hoell HV, Doyen JT, Purcell AH (1998). Introduction to Insect Biology and Diversity (2nd ed.). Oxford University Press. pp. 407–409. ISBN 978-0-19-510033-4.
- ↑ Harbison CW (2008). Ecology and Evolution of Transmission in Feather-feeding Lice (Phthiraptera: Ischnocera). Department of Biology (Doctoral thesis). University of Utah. pp. 83–87. ISBN 978-0-549-46429-7.
{{cite thesis}}: CS1 maint: date and year (link) - 1 2 3 4 5 Capinera JL (2008). Encyclopedia of Entomology. Springer Science & Business Media. pp. 838–844. ISBN 978-1-4020-6242-1.
- ↑ Gullan PJ, Cranston PS (2014). The Insects: An Outline of Entomology. Wiley. pp. 41–42. ISBN 978-1-118-84615-5.
- ↑ Smith V. "Phthiraptera: Summary". Phthiraptera.info. Retrieved 25 October 2015.
- ↑ Mumcuoglu KY (1999). "Prevention and treatment of head lice in children". Paediatric Drugs. 1 (3): 211–8. doi:10.2165/00128072-199901030-00005. PMID 10937452. S2CID 13547569.
- ↑ Rózsa (1997). "Patterns in the abundance of avian lice (Phthiraptera: Amblycera, Ischnocera)" (PDF). Journal of Avian Biology. 28 (3): 249–254. doi:10.2307/3676976. JSTOR 3676976.
- ↑ Rékási J, Rozsa L, Kiss BJ (1997). "Patterns in the distribution of avian lice (Phthiraptera: Amblycera, Ischnocera)" (PDF). Journal of Avian Biology. 28 (2): 150–156. CiteSeerX 10.1.1.506.730. doi:10.2307/3677308. JSTOR 3677308.
- ↑ Felsõ B, Rózsa L (August 2006). "Reduced taxonomic richness of lice (Insecta: Phthiraptera) in diving birds". The Journal of Parasitology. 92 (4): 867–9. doi:10.1645/ge-849.1. PMID 16995408. S2CID 31000581.
- ↑ Felső B, Rózsa L (2007). "Diving behaviour reduces genera richness of lice (Insecta: Phthiraptera) of mammals" (PDF). Acta Parasitologica. 52: 82–85. doi:10.2478/s11686-007-0006-3. S2CID 38683871.
- ↑ Møller AP, Rózsa L (January 2005). "Parasite biodiversity and host defenses: chewing lice and immune response of their avian hosts". Oecologia. 142 (2): 169–76. Bibcode:2005Oecol.142..169M. doi:10.1007/s00442-004-1735-8. PMID 15503162. S2CID 12992951.
- ↑ Møller AP, Erritzøe J, Rózsa L (June 2010). "Ectoparasites, uropygial glands and hatching success in birds". Oecologia. 163 (2): 303–11. Bibcode:2010Oecol.163..303M. doi:10.1007/s00442-009-1548-x. PMID 20043177. S2CID 11433594.
- 1 2 Ròzsa L (November 1993). "Speciation patterns of ectoparasites and "straggling" lice". International Journal for Parasitology. 23 (7): 859–64. doi:10.1016/0020-7519(93)90050-9. PMID 8314369.
- ↑ Paterson AM, Palma RL, Gray RD (1999). "How Frequently Do Avian Lice Miss the Boat? Implications for Coevolutionary Studies". Systematic Biology. 48: 214–223. doi:10.1080/106351599260544.
- ↑ MacLeod CJ, Paterson AM, Tompkins DM, Duncan RP (April 2010). "Parasites lost - do invaders miss the boat or drown on arrival?". Ecology Letters. 13 (4): 516–27. doi:10.1111/j.1461-0248.2010.01446.x. PMID 20455925.
- ↑ Rózsa L, Rékási J, Reiczigel J (1996). "Relationship of host coloniality to the population ecology of avian lice (Insecta: Phthiraptera)" (PDF). Journal of Animal Ecology. 65 (2): 242–248. doi:10.2307/5727. JSTOR 5727.
- ↑ Brown CR, Brown MB, Rannala B (1995). "Ectoparasites reduce long-term survivorship of their avian host" (PDF). Proceedings of the Royal Society of London B. 262 (1365): 313–319. doi:10.1098/rspb.1995.0211. S2CID 13869042.
- ↑ Barlett CM (1993). "Lice (Amblycera and Ischnocera) as vectors of Eulimdana spp. (Nematoda: Filarioidea) in Charadriiform birds and the necessity of short reproductive periods in adult worms". Journal of Parasitology. 75 (1): 85–91. doi:10.2307/3283282. JSTOR 3283282.
- ↑ Booth DT, Clayton DH, Block BA (1993). "Experimental demonstration of the energetic cost of parasitism in free-ranging hosts" (PDF). Proceedings of the Royal Society of London B. 253 (1337): 125–129. Bibcode:1993RSPSB.253..125B. doi:10.1098/rspb.1993.0091. S2CID 85731062. Archived from the original (PDF) on 2010-06-10. Retrieved 2010-08-14.
- ↑ Clayton (1990). "Mate choice in experimentally parasitized rock doves: lousy males lose" (PDF). American Zoologist. 30 (2): 251–262. doi:10.1093/icb/30.2.251. Archived from the original (PDF) on 2010-06-10. Retrieved 2010-08-14.
- ↑ Garamszegi LZ, Heylen D, Møller AP, Eens M, De Lope F (2005). "Age-dependent health status and song characteristics". Behavioral Ecology. 16 (3): 580–591. doi:10.1093/beheco/ari029.
- ↑ Light JE, Smith VS, Allen JM, Durden LA, Reed DL (September 2010). "Evolutionary history of mammalian sucking lice (Phthiraptera: Anoplura)". BMC Evolutionary Biology. 10 (1): 292. doi:10.1186/1471-2148-10-292. PMC 2949877. PMID 20860811.
- 1 2 Wappler T, Smith VS, Dalgleish RC (August 2004). "Scratching an ancient itch: an Eocene bird louse fossil". Proceedings. Biological Sciences. 271 Suppl 5 (suppl_5): S255-8. doi:10.1098/rsbl.2003.0158. PMC 1810061. PMID 15503987.
- ↑ Rasnitsyn AP, Zherikhin VV (1999). "First fossil chewing louse from the lower Cretaceous of Baissa, Transbaikalia (Insecta, Pediculida= Phthiriaptera, Saurodectidae fam. n.)". Russian Entomological Journal. 8 (4): 253–5.
- ↑ Ax P (2013). Multicellular Animals: Volume II: The Phylogenetic System of the Metazoa. Springer Science & Business Media. pp. 303–307. ISBN 978-3-662-10396-8.
- 1 2 Smith V. "Phthiraptera.info". International Society of Phthirapterists. Retrieved 25 October 2015.
- ↑ Banks JC, Paterson AM (2004). "A penguin-chewing louse (Insecta : Phthiraptera) phylogeny derived from morphology". Invertebrate Systematics. 18 (1): 89–100. doi:10.1071/IS03022. S2CID 53516244.
- 1 2 3 4 Reed DL, Light JE, Allen JM, Kirchman JJ (March 2007). "Pair of lice lost or parasites regained: the evolutionary history of anthropoid primate lice". BMC Biology. 5 (7): 7. doi:10.1186/1741-7007-5-7. PMC 1828715. PMID 17343749.
- 1 2 Parry W (7 November 2013). "Lice Reveal Clues to Human Evolution". LiveScience. Retrieved 25 October 2015.
- 1 2 Kittler R, Kayser M, Stoneking M (August 2003). "Molecular evolution of Pediculus humanus and the origin of clothing". Current Biology. 13 (16): 1414–7. doi:10.1016/S0960-9822(03)00507-4. PMID 12932325. S2CID 15277254.
- ↑ Shao R, Zhu XQ, Barker SC, Herd K (2012). "Evolution of extensively fragmented mitochondrial genomes in the lice of humans". Genome Biology and Evolution. 4 (11): 1088–101. doi:10.1093/gbe/evs088. PMC 3514963. PMID 23042553.
- 1 2 Light JE, Allen JM, Long LM, Carter TE, Barrow L, Suren G, et al. (December 2008). "Geographic distributions and origins of human head lice (Pediculus humanus capitis) based on mitochondrial data". The Journal of Parasitology. 94 (6): 1275–81. doi:10.1645/GE-1618.1. PMID 18576877. S2CID 9456340.
- ↑ Kowalski TJ, Agger WA (January 2009). "Art supports new plague science". Clinical Infectious Diseases. 48 (1): 137–8. doi:10.1086/595557. PMID 19067623.
- ↑ Harvey, Katherine (2019-04-09). "Medieval people were surprisingly clean (apart from the clergy)". Aeon. Retrieved 2022-10-13.
- ↑ Elliott L (2004). Clothing in the Middle Ages. Crabtree. p. 29. ISBN 978-0-7787-1351-7.
- ↑ Hooke R. "Microscopic view of a louse". The Royal Society. Retrieved 17 October 2015.
- ↑ Sarasohn LT (2010). The Natural Philosophy of Margaret Cavendish: Reason and Fancy During the Scientific Revolution. JHU Press. pp. 165–167. ISBN 978-0-8018-9443-5.
The Bear-men were to be her Experimental Philosophers, the Bird-men her Astronomers, the Fly- Worm- and Fish-men her Natural Philosophers, the Ape-men her Chymists, the Satyrs her Galenick Physicians, the Fox-men her Politicians, the Spider- and Lice-men her Mathematicians, the Jackdaw- Magpie- and Parrot-men her Orators and Logicians, the Gyants her Architects, &c.
- ↑ Cavendish M (1668). The Description of a New World, Called the Blazing-World. A. Maxwell.
- ↑ Zinsser H (2007) [1935]. Rats, Lice and History. Transaction Publishers. ISBN 978-1-4128-0672-5.
- ↑ Altschuler DZ (1990). "Zinsser, Lice and History". HeadLice.org. Retrieved 19 October 2015.
- ↑ Evans RJ. "The Great Unwashed". Gresham College. Retrieved 17 October 2015.
- ↑ Weinstein P (26 February 2013). "'The Great Unwashed': Entomophobia/Delusionary Parasitosis; Illusionary Parasitosis". The Great Plagues: Epidemics in History from the Middle Ages to the Present Day. University of Sydney Department of Medical Entomology. Archived from the original on 17 May 2016. Retrieved 17 October 2015.
- ↑ Kirkness EF, Haas BJ, Sun W, Braig HR, Perotti MA, Clark JM, et al. (July 2010). "Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle". Proceedings of the National Academy of Sciences of the United States of America. 107 (27): 12168–73. Bibcode:2010PNAS..10712168K. doi:10.1073/pnas.1003379107. PMC 2901460. PMID 20566863.
- ↑ Pittendrigh BR, Berenbaum MR, Seufferheld MJ, Margam VM, Strycharz JP, Yoon KS, et al. (March 2011). "Simplify, simplify: Lifestyle and compact genome of the body louse provide a unique functional genomics opportunity". Communicative & Integrative Biology. 4 (2): 188–91. doi:10.4161/cib.4.2.14279. PMC 3104575. PMID 21655436.
- ↑ White W (1859). Notes & Queries. Oxford University Press. pp. 275–276.
- ↑ Pierce H (2004). "Unseemly pictures: political graphic satire in England, c. 1600-c. 1650" (PDF). University of York.
- ↑ Joyce J (1939). Finnegans Wake. Faber. p. 180.
- ↑ Trafzer CE (2015). A Chemehuevi Song: The Resilience of a Southern Paiute Tribe. University of Washington Press. pp. 22–25. ISBN 978-0-295-80582-5.
- 1 2 Carroll J. "Songs of Clare: The Kilkenny Louse House". Clare Library. Retrieved 19 October 2015.
- ↑ Scott B (2013). "My Colleen by the Shore" (PDF). Veteran. Retrieved 25 October 2015.
- ↑ Twinn CR (1942). Insect Life in the Poetry and Drama of England: With Special Reference to Poetry (PhD thesis). University of Ottawa. hdl:10393/21088.
- ↑ "Woodlouse". Oxford English Dictionary (Online ed.). Oxford University Press. (Subscription or participating institution membership required.)
External links
- National Pesticide Information Center – Understanding and Controlling Lice
- body and head lice on the University of Florida/Institute of Food and Agricultural Sciences Featured Creatures Web site
- crab louse on the University of Florida/Institute of Food and Agricultural Sciences Featured Creatures Web site
- Pediculus humanus capitis head louse facts, myths, life cycle at MetaPathogen
- Parasitic Insects, Mites and Ticks: Genera of Medical and Veterinary Importance Wikibooks