 | |
| Clinical data | |
|---|---|
| Other names | Methylhomatropine bromide |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.168 |
| Chemical and physical data | |
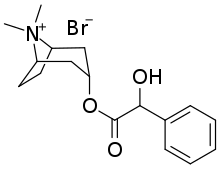
| Formula | C17H24BrNO3 |
| Molar mass | 370.287 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Homatropine methylbromide (INN; also known as methylhomatropine bromide) is a quaternary ammonium salt of methylhomatropine. It is a peripherally acting anticholinergic medication that inhibits muscarinic acetylcholine receptors and thus the parasympathetic nervous system. It does not cross the blood–brain barrier. It is used to effectively relieve intestinal spasms and abdominal cramps, without producing the adverse effects of less specific anticholinergics.[1] It is used, in addition to papaverine, as a component of mild drugs that help "flush" the bile.
Certain preparations of drugs such as hydrocodone are mixed with a small, sub-therapeutic amount of homatropine methylbromide to discourage intentional overdose.[2]
Contraindications
- Untreated glaucoma
- Myasthenia gravis
- Severe heart failure
- Thyrotoxicosis
See also
References
- ↑ "Paratropina (Homatropine Methylbromide)". Archived from the original on 2017-04-29. Retrieved 2016-02-18.
- ↑ "Hydromet (Hydrocodone Bitartrate and Homatropine Methylbromide)". DailyMed. NIH.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.