 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
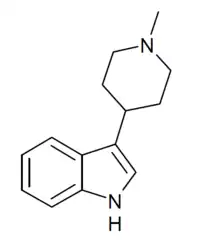
| Formula | C14H18N2 |
| Molar mass | 214.312 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
SN-22 is a chemical compound which acts as a moderately selective agonist at the 5-HT2 family of serotonin receptors, with a Ki of 19nM at 5HT2 subtypes vs 514 nM at 5-HT1A receptors.[1] Many related derivatives are known, most of which are ligands for 5-HT1A, 5-HT6 or dopamine D2 receptors or show SSRI activity.[2][3][4][5][6]
See also
References
- ↑ Taylor EW, Nikam SS, Lambert G, Martin AR, Nelson DL (July 1988). "Molecular determinants for recognition of RU 24969 analogs at central 5-hydroxytryptamine recognition sites: use of a bilinear function and substituent volumes to describe steric fit". Molecular Pharmacology. 34 (1): 42–53. PMID 3393140.
- ↑ Agarwal A, Pearson PP, Taylor EW, Li HB, Dahlgren T, Herslöf M, et al. (December 1993). "Three-dimensional quantitative structure-activity relationships of 5-HT receptor binding data for tetrahydropyridinylindole derivatives: a comparison of the Hansch and CoMFA methods". Journal of Medicinal Chemistry. 36 (25): 4006–14. doi:10.1021/jm00077a003. PMID 8258822.
- ↑ Cole DC, Ellingboe JW, Lennox WJ, Mazandarani H, Smith DL, Stock JR, et al. (January 2005). "N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 15 (2): 379–83. doi:10.1016/j.bmcl.2004.10.064. PMID 15603958.
- ↑ Deskus JA, Epperson JR, Sloan CP, Cipollina JA, Dextraze P, Qian-Cutrone J, et al. (June 2007). "Conformationally restricted homotryptamines 3. Indole tetrahydropyridines and cyclohexenylamines as selective serotonin reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters. 17 (11): 3099–104. doi:10.1016/j.bmcl.2007.03.040. PMID 17391962.
- ↑ Mattsson C, Andreasson T, Waters N, Sonesson C (November 2012). "Systematic in vivo screening of a series of 1-propyl-4-arylpiperidines against dopaminergic and serotonergic properties in rat brain: a scaffold-jumping approach". Journal of Medicinal Chemistry. 55 (22): 9735–50. doi:10.1021/jm300975f. PMID 23043306.
- ↑ US 6046215, "Inhibition of serotonin reuptake"
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.