 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.220 |
| Chemical and physical data | |
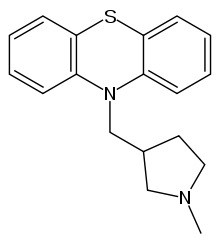
| Formula | C18H20N2S |
| Molar mass | 296.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Methdilazine (Dilosyn, Tacaryl) is a first-generation antihistamine with anticholinergic properties of the phenothiazine class.
Synthesis
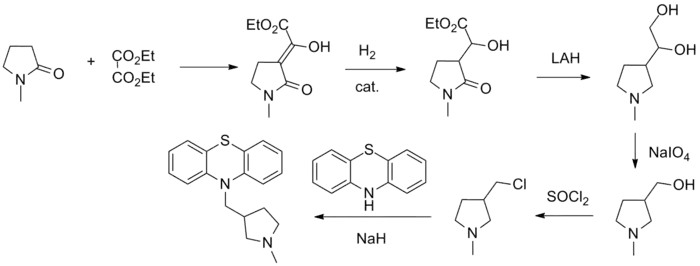
Methdilazine synthesis:[1] R. F. Feldkamp and Y. H. Wu; Mead Johnson & Company; U.S. Patent 2,945,855 (1960).
See also
References
- ↑ L. W. Marsch and R. Peterson, Arzneimittel Forsch., 9, 715 (1959).
- Rani Basu L, Mazumdar K, Dutta N, Karak P, Dastidar S (2005). "Antibacterial property of the antipsychotic agent prochlorperazine, and its synergism with methdilazine". Microbiol Res. 160 (1): 95–100. doi:10.1016/j.micres.2004.10.002. PMID 15782943.
- Chattopadhyay D, Mukherjee T, Pal P, Saha B, Bhadra R (1998). "Altered membrane permeability as the basis of bactericidal action of methdilazine". J Antimicrob Chemother. 42 (1): 83–6. doi:10.1093/jac/42.1.83. PMID 9700532.
- Chattopadhyay D, Dastidar S, Chakrabarty A (1988). "Antimicrobial properties of methdilazine and its synergism with antibiotics and some chemotherapeutic agents". Arzneimittelforschung. 38 (7): 869–72. PMID 2905130.
The ring-contracted analog, methdilazine shows only very weak activity as a tranquilizer; instead, that agent constitutes an important antihistamine.
| Benzimidazoles (*) | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
| H1 |
| ||||
|---|---|---|---|---|---|
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
| Classes | |
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.