 | |
 | |
| Names | |
|---|---|
| IUPAC name
Oxovanadium(2+) sulfate | |
| Other names
Basic vanadium(IV) sulfate Vanadium(IV) oxide sulfate Vanadium(IV) oxysulfate | |
| Identifiers | |
| |
| ECHA InfoCard | 100.044.214 |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| H10O10SV | |
| Molar mass | 253.07 g·mol−1 |
| Appearance | Blue solid |
| Melting point | 105 °C (221 °F; 378 K) decomposes |
| Soluble | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| Flash point | Non-flammble |
| Related compounds | |
Other anions |
Vanadyl chloride Vanadyl nitrate |
Other cations |
Vanadium(III) sulfate |
Related compounds |
Vanadyl acetylacetonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |

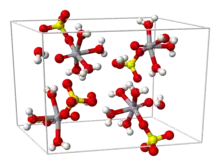
Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".[1]
Vanadyl sulfate is an intermediate in the extraction of vanadium from petroleum residues, one commercial source of vanadium.[2]
Synthesis, structure, and reactions
Vanadyl sulfate is most commonly obtained by reduction of vanadium pentoxide with sulfur dioxide:
- V2O5 + 7 H2O + SO2 + H2SO4 → 2 [V(O)(H2O)4]SO4
From aqueous solution, the salt crystallizes as the pentahydrate, the fifth water is not bound to the metal in the solid. Viewed as a coordination complex, the ion is octahedral, with oxo, four equatorial water ligands, and a monodentate sulfate.[1][3] The trihydrate has also been examined by crystallography.[4] A hexahydrate exists below 13.6 °C (286.8 K).[5] Two polymorphs of anhydrous VOSO4 are known.[6]
The V=O bond distance is 160 pm, about 50 pm shorter than the V–OH2 bonds. In solution, the sulfate ion dissociates rapidly.
Being widely available, vanadyl sulfate is a common precursor to other vanadyl derivatives, such as vanadyl acetylacetonate:[7]
- [V(O)(H2O)4]SO4 + 2 C5H8O2 + Na2CO3 → [V(O)(C5H7O2)2] + Na2SO4 + 5 H2O + CO2
In acidic solution, oxidation of vanadyl sulfate gives yellow-coloured vanadyl(V) derivatives. Reduction, e.g. by zinc, gives vanadium(III) and vanadium(II) derivatives, which are characteristically green and violet, respectively.
Occurrence in nature
Like most water-soluble sulfates, vanadyl sulfate is only rarely found in nature. Anhydrous form is pauflerite,[8] a mineral of fumarolic origin. Hydrated forms, also rare, include hexahydrate (stanleyite), pentahydrates (minasragrite, orthominasragrite,[9] and anorthominasragrite) and trihydrate - bobjonesite.[10]
Medical research
Vanadyl sulfate is a component of food supplements and experimental drugs. Vanadyl sulfate exhibits insulin-like effects.[11]
Vanadyl sulfate has been extensively studied in the field of diabetes research as a potential means of increasing insulin sensitivity. No evidence indicates that oral vanadium supplementation improves glycaemic control.[12][13] Treatment with vanadium often results in gastrointestinal side-effects, primarily diarrhea.
Vanadyl sulfate is also marketed as a health supplement, often for bodybuilding. Deficiencies in vanadium result in reduced growth in rats.[14] Its effectiveness for bodybuilding has not been proven; some evidence suggests that athletes who take it are merely experiencing a placebo effect.[15]
References
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 1157. ISBN 978-0-08-022057-4.
- ↑ Günter Bauer; Volker Güther; Hans Hess; Andreas Otto; Oskar Roidl; Heinz Roller; Siegfried Sattelberger (2005). "Vanadium and Vanadium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_367. ISBN 3-527-30673-0.
- ↑ Tachez, M.; Theobald, F.R. (1980). "Structure du Sulfate de Vanadyle Pentahydrate VO(H2O)5SO4 beta (variete orthorhombique)". Acta Crystallographica B. B36 (8): 1757–p1761. doi:10.1107/S0567740880007170.
- ↑ Tachez, M.; Theobald, F. R. (1980). "Liaisons hydrogene dans les cristaux de sulfate de vanadyle trihydrate VOSO4(H2O)3: Comparaison structurale de quatre sulfates de vanadyle hydrate". Acta Crystallographica B. 36: 2873–2880. doi:10.1107/S056774088001045X.
- ↑ M. Tachez, F. Theobald, G. Trouillot. Crystal data for vanadyl sulphate hexahydrate VOSO4.6H2O. J. Appl. Crystallogr. (1976). 9, 246
- ↑ Boghosian, S.; Eriksen, K.M.; Fehrmann, R.; Nielsen, K. (1995). "Synthesis, Crystal Structure Redetermination and Vibrational Spectra of beta- VOSO4". Acta Chemica Scandinavica. 49: 703–708. doi:10.3891/acta.chem.scand.49-0703.Longo, J. M.; Arnott, R. J. (1970). "Structure and magnetic properties of VOSO4". Journal of Solid State Chemistry. 1 (3–4): 394–p398. Bibcode:1970JSSCh...1..394L. doi:10.1016/0022-4596(70)90121-0.
- ↑ Bryant, Burl E.; Fernelius, W. Conard (1957), "Vanadium(IV) Oxy(acetylacetonate)", Inorg. Synth., Inorganic Syntheses, 5: 113–16, doi:10.1002/9780470132364.ch30, ISBN 978-0-470-13236-4
- ↑ Krivovichev, S. V.; Vergasova, L. P.; Britvin, S. N.; Filatov, S. K.; Kahlenberg, V.; Ananiev, V. V. (1 August 2007). "Pauflerite, -VO(SO4), a New Mineral Species from the Tolbachik Volcano, Kamchatka Peninsula, Russia". The Canadian Mineralogist. 45 (4): 921–927. doi:10.2113/gscanmin.45.4.921.
- ↑ Hawthorne, F. C.; Schindler, M.; Grice, J. D.; Haynes, P. (1 October 2001). "Orthominasragrite, V4+O(SO4)(H2O)5, A New Mineral Species from Temple Mountain, Emery County, Utah, U.A.A.". The Canadian Mineralogist. 39 (5): 1325–1331. doi:10.2113/gscanmin.39.5.1325.
- ↑ Schindler, M.; Hawthorne, F. C.; Huminicki, D. M.C.; Haynes, P.; Grice, J. D.; Evans, H. T. (1 February 2003). "Bobjonesite, V4+ O (So4) (H2O)3, A New Mineral Species from Temple Mountain, Emery County, Utah, U.s.a.". The Canadian Mineralogist. 41 (1): 83–90. doi:10.2113/gscanmin.41.1.83.
- ↑ Crans, D. C.; Trujillo, A. M.; Pharazyn, P. S.; Cohen, M. D. (2011). "How environment affects drug activity: Localization, compartmentalization and reactions of a vanadium insulin-enhancing compound, dipicolinatooxovanadium(V)". Coord. Chem. Rev. 255 (19–20): 2178–2192. doi:10.1016/j.ccr.2011.01.032.
- ↑ Yeh, Gloria Y.; Eisenberg, David M.; Kaptchuk, Ted J.; Phillips, Russell S. (2003). "Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes". Diabetes Care. 26 (4): 1277–1294. doi:10.2337/diacare.26.4.1277. PMID 12663610.
- ↑ Smith, D.M.; Pickering, R.M.; Lewith, G.T. (31 January 2008). "A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus". QJM. 101 (5): 351–358. doi:10.1093/qjmed/hcn003. PMID 18319296.
- ↑ Schwarz, Klaus; Milne, David B. (1971). "Growth Effects of Vanadium in the Rat". Science. 174 (4007): 426–428. Bibcode:1971Sci...174..426S. doi:10.1126/science.174.4007.426. JSTOR 1731776. PMID 5112000. S2CID 24362265.
- ↑ Talbott, Shawn M.; Hughes, Kerry (2007). "Vanadium". The Health Professional's Guide to Dietary Supplements. Lippincott Williams & Wilkins. pp. 419–422. ISBN 978-0-7817-4672-4.