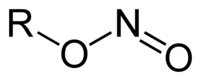亚硝酸酯
性质
亚硝酸甲酯、亚硝酸乙酯在通常条件下为气体。较低级的亚硝酸酯为有特殊果香的挥发性液体。
不稳定,缓慢分解为氮氧化物、水、相应的醇,以及相应的醛的聚合产物。[1]
和硝酸酯类似,亚硝酸酯也是一类药物。
性质
1、亚硝酸酯的冰醋酸溶液中存在乙酰硝酸酯(CH3COONO2),乙酰硝酸酯是温和的硝化试剂,可对其他化合物进行硝化,它也存在于较为传统的硝酸-乙酸酐硝化系统中。
2、亚硝酸正丁酯和氨可将苯胲转化为其N-亚硝基衍生物铜铁试剂。[2]吡咯烷也可与亚硝酸乙酯生成相应的N-NO衍生物。[3]
3、在酸或碱催化下,亚硝酸酯可与較強的碳负离子反应,在α位引入肟基(=NOH)。例如,用丁酮、亚硝酸乙酯、盐酸反应,然后用羟胺磺酸钠处理,则最后可以得到丁二酮肟;[4]如果以苯酰甲基氯为原料,亚硝酸正丁酯为试剂,在酸性条件下反应,则可得ω-氯肟基苯乙酮;[5]用亚硝酸甲酯在氢氧化钠中处理苯乙腈,则可得α-肟基苯乙腈。[6]
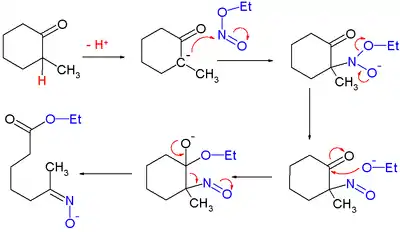
Woodward和Doering的奎宁全合成也用到了上述反应:[7]
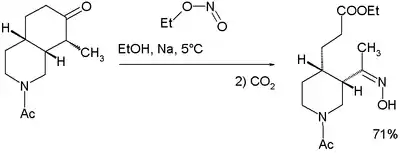 他们认为反应机理如下:
他们认为反应机理如下:
参考资料
- W. A. Noyes. "n-butyl nitrite (页面存档备份,存于)". Org. Synth.; Coll. Vol. 16: 7. (1936); Coll. Vol. 2: 108. (1943).
- C. S. Marvel. "Cupferron (页面存档备份,存于)". Org. Synth.; Coll. Vol. 1: 177. (1941); Coll. Vol. 4: 19. (1925).
- D. Enders, R. Pieter, B. Renger, and D. Seebach. "Nucleophilic α-sec-Aminoalkylation: 2-(Diphenylhydroxymethyl)pyrrolidine (页面存档备份,存于)". Org. Synth.; Coll. Vol. 6: 542. (1988); Coll. Vol. 58: 113. (1978).
- W. L. Semon, V. R. Damerell. "Dimethylglyoxime (页面存档备份,存于)". Org. Synth.; Coll. Vol. 2: 204. (1943); Coll. Vol. 10: 22. (1930).
- Nathan Levin, Walter H. Hartung. "ω-Chloroisonitrosoacetophenone (页面存档备份,存于)". Org. Synth.; Coll. Vol. 3: 191. (1955); Coll. Vol. 24: 25. (1944).
- Masumi Itoh, Daijiro Hagiwara, Takashi Kamiya1. "A New Reagent for tert-Butoxycarbonylation: 2-tert-Butoxycarbonyloxyimino-2-phenylacetonitrile (页面存档备份,存于)". Org. Synth.; Coll. Vol. 6: 199. (1988); Coll. Vol. 59: 95. (1979).
- Woodward, R. B.; Doering, W. E. . Journal of the American Chemical Society. 1945-05, 67 (5): 860–874 [2020-12-07]. ISSN 0002-7863. doi:10.1021/ja01221a051. (原始内容存档于2020-05-27) (英语).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.