偶联反应
偶联反应,也写作耦合反應、偶合反应或耦联反应,是两化学实体(或单位)结合生成一分子的有机化学反应。狭义的偶联反应是涉及有机金属催化剂的碳-碳键形成反应,根据类型的不同,又可分为交叉偶联和自身偶联反应。在偶联反应中有一类重要的反应,RM(R=有机片段;M=主基团中心)与R'X的有机卤素化合物反应,形成有新碳-碳键的产物R-R'[1][2]。
根岸英一、铃木章与理查德·赫克开发钯催化偶联反应,贡献突出,共同獲授2010年度诺贝尔化学奖[3][4]。
偶联反应大体可分为两种类型:
机理

偶联机理通常起始于有机卤代烃和催化剂氧化加成;第二步是另一分子与其转移金属化,将待偶联的两分子接于同一金属中心;最后一步是还原消除,待偶联的两分子结合在一起形成新分子并再生催化剂。不饱和的有机基团在加合一步速度更快,通常易于偶联。中间体通常不倾向β-氢消除。[5]
一项计算化学研究中表明,不饱和有机基团更易于在金属中心偶联。[6]还原消除的速率高低如下:
乙烯基-乙烯基>苯基-苯基>炔基-炔基>烷基-烷基
不对称的R-R′形式偶联反应,其活化能垒与反应能量与相应的对称偶联反应R-R与R′-R′的平均值相近,如:乙烯基-乙烯基>乙烯基-烷基>烷基-烷基。
另一种假说认为,水溶液偶联反应其实是通过自由基机理,而不是金属-参与机理。[7]
类型
常见偶联反应有:
| 反应名称 | 发现年代 | 反应物A | 反应物B | 类型 | 催化剂 | 备注 | |||
| 武兹反应(Wurtz reaction) | 1855 | R-X | sp³ | R-X | sp³ | 自身 | 以鈉消除反应物的卤原子 | ||
| 格拉泽偶联反应(Glaser coupling) | 1869 | RC≡CH | sp | RC≡CH | sp | 自身 | Cu | 氧气作H受体 | |
| 乌尔曼反应(Ullmann reaction) | 1901 | Ar-X | sp² | Ar-X | sp² | 自身 | Cu | 高温 | 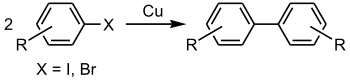 |
| 冈伯格-巴克曼反应 | 1924 | Ar-H | sp² | Ar-N2X | sp² | 自身 | 需碱参与 | 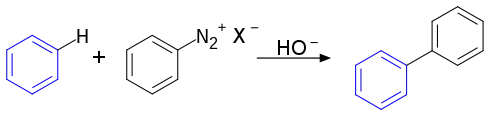 | |
| Cadiot-Chodkiewicz偶联反应 | 1957 | RC≡CH | sp | RC≡CX | sp | 交叉 | Cu | 需碱参与 |  |
| Castro-Stephens偶联反应 | 1963 | RC≡CH | sp | Ar-X | sp² | 交叉 | Cu | ||
| 吉尔曼试剂偶联反应(Gilman reagent coupling) | 1967 | R2CuLi | R-X | 交叉 | |||||
| Cassar反应 | 1970 | 烯烃 | sp² | R-X | sp³ | 交叉 | Pd | 需碱参与 | |
| 熊田偶联反应(Kumada coupling) | 1972 | Ar-MgBr | sp²/sp³ | Ar-X | sp² | 交叉 | Pd或Ni | 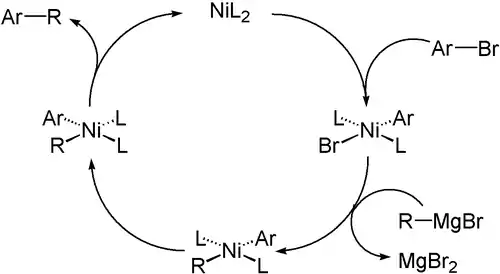 | |
| 赫克反应(Heck reaction) | 1972 | 烯烃 | sp² | R-X | sp² | 交叉 | Pd | 需碱参与 | |
| 薗头偶联反应(Sonogashira coupling) | 1975 | RC≡CH | sp | R-X | sp³/sp² | 交叉 | Pd和Cu | 需碱参与 |  |
| 根岸偶联反应(Negishi coupling) | 1977 | R-Zn-X | sp³/sp²/sp | R-X | sp³/sp² | 交叉 | Pd或Ni | ||
| 施蒂勒反应(Stille coupling) | 1978 | R-SnR3 | sp³/sp²/sp | R-X | sp³/sp² | 交叉 | Pd | ||
| 铃木反应(Suzuki reaction) | 1979 | R-B(OR)2 | sp² | R-X | sp³/sp² | 交叉 | Pd | 需碱参与 | |
| Hiyama偶联反应 | 1988 | R-SiR3 | sp² | R-X | sp³/sp² | 交叉 | Pd | 需碱参与 | 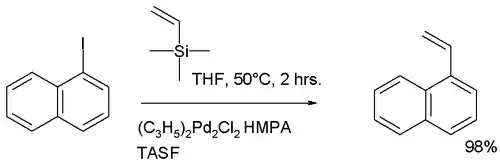 |
| Buchwald–Hartwig偶联反应 | 1994 | R2N-R SnR3 | sp | R-X | sp² | 交叉 | Pd | N-C偶联反应 | |
| 福山偶联反应(Fukuyama coupling) | 1998 | RCO(SEt) | sp2 | R-Zn-I | sp3 | 交叉 | Pd | 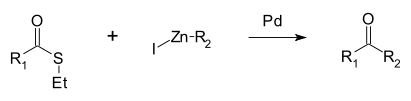 | |
| 偶联反应一览. 参考文献见子页面 | |||||||||
杂项反应
一种基于芳基卤代烃和全氟代苯的钯-催化偶联反应由基思·法纽(Keith Fagnou)和其同事所报道。对于这种缺电子的芳环来讲,这类官能团化反应很不寻常。[17]

Fluoroarene coupling
参见
参考资料
- Organic Synthesis using Transition Metals Rod Bates ISBN 978-1-84127-107-1
- New Trends in Cross-Coupling: Theory and Applications Thomas Colacot (Editor) 2014 ISBN 978-1-84973-896-5
- . NobelPrize.org. 2010-10-06 [2010-10-06]. (原始内容存档于2012-10-26).
- Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize Dr. Carin C. C. Johansson Seechurn, Dr. Matthew O. Kitching, Dr. Thomas J. Colacot, Prof. Victor Snieckus Angew. Chem. Int. Ed. 2012, 51, 5062-5085. doi:10.1002/anie.201107017
- Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X
- V. P. Ananikov, D. G. Musaev, K. Morokuma, “Theoretical Insight into the C-C Coupling Reactions of the Vinyl, Phenyl, Ethynyl, and Methyl Complexes of Palladium and Platinum” Organometallics 2005, 24, 715. doi:10.1021/om0490841
- Benny Bogoslavsky, Ophir Levy, Anna Kotlyar, Miri Salem, Faina Gelman and Avi Bino. . Angewandte Chemie International Edition. 2012, 51 (1): 90–94. PMID 22031005. doi:10.1002/anie.201103652.
- Cobalt-Catalyzed Cross-Coupling Reactions Grard Cahiez and Alban Moyeux Chem. Rev., 2010, 110 (3), pp 1435–1462 Publication Date (Web): February 11, 2010 (Review) doi:10.1021/cr9000786
- Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts Lunxiang Yin and Jürgen Liebscher Chem. Rev., 2007, 107 (1), pp 133–173 Publication Date (Web): December 21, 2006 (Article) doi:10.1021/cr0505674
- Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners Ranjan Jana, Tejas P. Pathak, and Matthew S. Sigman Chem. Rev., 2011, 111 (3), pp 1417–1492 doi: 10.1021/cr100327p
- Efficient, Selective, and Recyclable Palladium Catalysts in Carbon−Carbon Coupling Reactions rpd Molnr Chem. Rev., 2011, 111 (3), pp 2251–2320 doi:10.1021/cr100355b
- Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds Norio. Miyaura, Akira. Suzuki Chem. Rev., 1995, 95 (7), pp 2457–2483 doi:10.1021/cr00039a007
- Diazonium Salts as Substrates in Palladium-Catalyzed Cross-Coupling Reactions Anna Roglans, Anna Pla-Quintana, and Marcial Moreno-Mañas Chem. Rev., 2006, 106 (11), pp 4622–4643 doi:10.1021/cr0509861
- Nickel-Catalyzed Cross-Couplings Involving Carbon−Oxygen Bonds Brad M. Rosen, Kyle W. Quasdorf, Daniella A. Wilson, Na Zhang, Ana-Maria Resmerita, Neil K. Garg, and Virgil Percec Chem. Rev., 2011, 111 (3), pp 1346–1416 doi:10.1021/cr100259t
- Selected Patented Cross-Coupling Reaction Technologies Jean-Pierre Corbet and Gérard Mignani Chem. Rev., 2006, 106 (7), pp 2651–2710 2006 (Article) doi:10.1021/cr0505268
- Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis Gwilherm Evano, Nicolas Blanchard and Mathieu Toumi Chem. Rev., 2008, 108 (8), pp 3054–3131 doi:10.1021/cr8002505
- M. Lafrance, C. N. Rowley, T. K. Woo and K. Fagnou. . J. Am. Chem. Soc. 2006, 128 (27): 8754–8756. PMID 16819868. doi:10.1021/ja062509l.
- R.H. Crabtree, The Organometallic Chemistry of the Transition Metals 4th Ed.
- Organotransition Metal Chemistry: From Bonding to Catalysis John Hartwig
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.