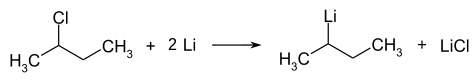
仲丁基锂
仲丁基锂,化学式 CH
3CHLiCH
2CH
3,简称sec-BuLi或s-BuLi,是一种有机锂化合物,为正丁基锂和叔丁基锂的异构体。这种有手性的有机锂试剂是有机合成中仲丁基碳阴离子的来源。[2]
| 仲丁基锂 | |
|---|---|
 | |
 | |
| IUPAC名 sec-Butyllithium | |
| 系统名 Butan-2-yllithium | |
| 英文名 | |
| 识别 | |
| CAS号 | 598-30-1 |
| PubChem | 102446 |
| ChemSpider | 10254345 |
| SMILES |
|
| InChI |
|
| InChIKey | VATDYQWILMGLEW-MHILWDCKAX |
| Beilstein | 3587206 |
| EINECS | 209-927-7 |
| 性质 | |
| 化学式 | C4H9Li |
| 摩尔质量 | 64.06 g·mol−1 |
| 外观 | 无色液体[1] |
| 沸点 | 90℃ (0.05 Torr)[2] |
| 溶解性 | 可溶于环己烷[3] |
| pKa | 51 |
| 相关物质 | |
| 相关化学品 | 正丁基锂 叔丁基锂 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
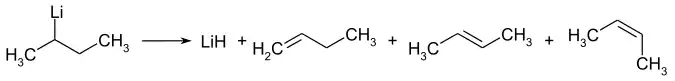
性质

用处
碳-锂键是高度极性的,所以其中的碳原子的亲核性和碱性很强。位阻更多的仲丁基锂的碱性比正丁基锂还强。这些性质可用于各种用途。举个例子,当有机化合物的C-H键酸性很弱,正丁基锂不能使用时,可以使用仲丁基锂。它也用于催化异戊二烯、丁二烯和苯乙烯的聚合反应。[1]
参考资料
- Entry on Butyllithium. at: Römpp Online. Georg Thieme Verlag, retrieved 2013-12-06.
- Ovaska, T. V. "s-Butyllithium" in Encyclopedia of Reagents for Organic Synthesis, 2001 John Wiley & Sons: New York. doi:10.1002/047084289X.rb397.
- 来源:Sigma-Aldrich Co., product no. 195596 (2011-04-23查阅).
- H. Gilman, F. W. Moore, O. Baine: Secondary and Tertiary Alkyllithium Compounds and Some Interconversion Reactions with Them. In: J. Am. Chem. Soc. 63, 1941, S. 2479–2482, doi:10.1021/ja01854a046.
- U. Wietelmann, R. J. Bauer: Lithium and Lithium Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim 2005, doi:10.1002/14356007.a15_393
- D. Plavsic, D. Srzic, L. Klasinc: Mass spectrometric investigations of alkyllithium compounds in the gas phase. In: J. Phys. Chem. 90, 1986, S. 2075–2080, doi:10.1021/j100401a020.
- S. Bywater, D. J. Worsfold: Alkyllithium anionic polymerization initiators in hydrocarbon solvents. In: J. Organomet. Chem. 10, 1967, S. 1–6.
- G. Fraenkel, M. Henrichs, M. Hewitt, B. M. Su: Structure and dynamic behavior of a chiral alkyllithium compound: 13C and 6Li NMR of sec-butyllithium. In: J. Am. Chem. Soc. 106, 1984, S. 255–256.
- W. Bauer, W. R. Winchester, P. von Schleyer: Monomeric organolithium compounds in tetrahydrofuran: tert-butyllithium, sec-butyllithium, supermesityllithium, mesityllithium, and phenyllithium. Carbon-lithium coupling constants and the nature of carbon-lithium bonding. In: Organometallics. 6, 1987, S. 2371–2379, doi:10.1021/om00154a017.
- W. H. Glaze, J. Lin, E. G. Felton: The Thermal Decomposition of sec.-Butyllithium. In: J. Org. Chem. 30, 1965, S. 1258–1259, doi:10.1021/jo01015a514.
- W. H. Glaze, J. Lin, E. G. Felton: The Pyrolysis of Unsolvated Alkyllithium Compounds. In: J. Org. Chem. 31, 1966, S. 2643–2645, doi:10.1021/jo01346a044.
、
扩展阅读
- Heinz G. O. Becker u. a.: Organikum. 21. Auflage. Wiley-VCH, Weinheim 2001, ISBN 3-527-29985-8.
- Christoph Elschenbroich: Organometallchemie. 5. Auflage. Teubner, Wiesbaden 2005, ISBN 3-519-53501-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.