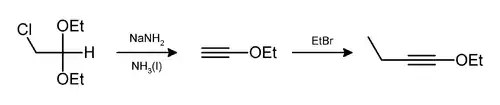氨基钠
氨基钠是一种无机化合物,化学式为NaNH2。室温下纯品为白色固体,试剂常带金属铁而呈灰色。氨基钠与水强烈反应,是有机合成中常用的强碱。
| 氨基钠 | |
|---|---|
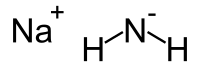 | |
 | |
 | |
| IUPAC名 Sodium amide | |
| 英文名 | |
| 别名 | 氨钠 |
| 识别 | |
| CAS号 | 7782-92-5 |
| PubChem | 24533 |
| ChemSpider | 22940 |
| SMILES |
|
| UN编号 | 1390 |
| EINECS | 231-971-0 |
| 性质 | |
| 化学式 | NaNH2 |
| 39.01 g·mol⁻¹ | |
| 外观 | 灰色粉末 纯品白色 |
| 密度 | 1.37 g/cm3 (白) |
| 熔点 | 210 °C |
| 沸点 | 400 °C |
| 溶解性(水) | 反应 |
| pKb | 38 |
| 结构 | |
| 配位几何 | 四面体 (Na, N) |
| 危险性 | |
| 欧盟分类 | 未列明 |
| NFPA 704 |
 2
3
3
|
| 闪点 | 4.44 °C |
| 自燃温度 | 450 °C |
| 相关物质 | |
| 其他阴离子 | 双(三甲硅基)氨基钠、肼基钠 |
| 其他阳离子 | 氨基钾、氨基銣、氨基銫 |
| 相关化学品 | 氨 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
制备及结构
氨基钠可由钠与氨气反应,[1] 或硝酸铁催化下钠与液氨的反应来制备。后者反应在氨沸点(约-33°C)时反应最快,也更常用:[2]
- 2 Na + 2 NH3 → 2 NaNH2 + H2
NaNH2为类盐固体,晶格中[3] 钠原子为四面体结构。[4] 溶于氨时,NaNH2溶液存在Na(NH3)6+和NH2−离子,可导电。
用途
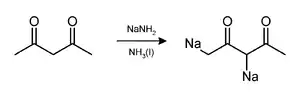
工业上,氨基钠是制取靛青染料、肼和氰化钠等工业品的原料。[5] 可用于干燥液氨或氨气,其液氨溶液也是有机化学中常用的强碱。氨基钠碱性很强而且亲核性很弱,但不易溶于大多数溶剂中,因而已被氢化钠、双(三甲硅基)氨基钠(NaHMDS)以及二异丙基氨基锂(LDA)等类似试剂所取代。
安全
氨基钠会与水猛烈反应生成氢氧化钠和氨,且在空气中可燃烧生成钠氧化物及二氧化氮:
氨基钠应当储存在密闭容器中,最好以氮气保护。当放置于密闭性不好的容器中时,氧气和水不充足,有可能生成爆炸性的氧化产物从而使固体泛黄棕色。此类试剂应当尽快销毁,方法为向氨基钠的烃类溶剂悬浮液中小心加入乙醇。
氨基钠可能会对皮肤、眼部及黏膜有強腐蚀性,应当留心空气中悬浮的氨基钠粉末。
参考文献
- Bergstrom, F. W. (1955). "Sodium amide (页面存档备份,存于)". Org. Synth. Coll. Vol. 3:778.
- Greenlee, K. W.; Henne, A. L. (1946). "Sodium Amide". Inorganic Syntheses 2:128–35.
- Zalkin, A.; Templeton, D. H. "The Crystal Structure Of Sodium Amide" Journal of Physical Chemistry 1956, Volume 60, pp 821 - 823. DOI: 10.1021/j150540a042
- Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- Merck Index (12th Edn.)
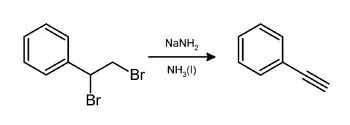
- Campbell, Kenneth N.; Campbell, Barbara K. (1950). "Phenylacetylene (页面存档备份,存于)". Org. Synth. 30:72; Coll. Vol. 4:763.
- Jones, E. R. H.; Eglinton, Geoffrey; Whiting, M. C.; Shaw, B. L. (1954). "Ethoxyacetylene (页面存档备份,存于)". Org. Synth. 34:46; Coll. Vol. 4:404.
Bou, Anna; Pericàs, Miquel A.; Riera, Antoni; Serratosa, Fèlix (1987). "Dialkoxyacetylenes: di-tert-butoxyethyne, a valuable synthetic intermediate (页面存档备份,存于)". Org. Synth. 65:68; Coll. Vol. 8:161.
Magriotis, Plato A.; Brown, John T. (1995). "Phenylthioacetylene (页面存档备份,存于)". Org. Synth. 72:252; Coll. Vol. 9:656.
Ashworth, P. J.; Mansfield, G. H.; Whiting, M. C. (1955). "2-Butyn-1-ol (页面存档备份,存于)". Org. Synth. 35:20; Coll. Vol. 4:128. - Newman, Melvin S.; Stalick, W. M. (1977). "1-Ethoxy-1-butyne (页面存档备份,存于)". Org. Synth. 57:65; 6:564.
- Salaun, J. R.; Champion, J.; Conia, J. M. (1977). "Cyclobutanone from methylenecyclopropane via oxaspiropentane (页面存档备份,存于)". Org. Synth. 57:36; Coll. Vol. 6:320.
- Nakamura, Masuharu; Wang, Xio Qun; Isaka, Masahiko; Yamago, Shigeru; Nakamura, Eiichi (2003). "Synthesis and (3+2)-cycloaddition of a 2,2-dialkoxy-1-methylenecyclopropane: 6,6-dimethyl-1-methylene-4,8-dioxaspiro(2.5)octane and cis-5-(5,5-dimethyl-1,3-dioxan-2-ylidene)hexahydro-1(2H)-pentalen-2-one (页面存档备份,存于)". Org. Synth. 80:144.
- Bottini, Albert T.; Olsen, Robert E. (1964). "N-Ethylallenimine (页面存档备份,存于)". Org. Synth. 44:53; Coll. Vol. 5:541.
- Skorcz, J. A.; Kaminski, F. E. (1968). "1-Cyanobenzocyclobutene (页面存档备份,存于)". Org. Synth. 48:55; Coll. Vol. 5:263.
- Saunders, J. H. (1949). "1-Ethynylcyclohexanol (页面存档备份,存于)". Org. Synth. 29:47; Coll. Vol. 3:416.
Peterson, P. E.; Dunham, M. (1977). "(Z)-4-Chloro-4-hexenyl trifluoroacetate (页面存档备份,存于)". Org. Synth. 57:26; Coll. Vol. 6:273.
Kauer, J. C.; Brown, M. (1962). "Tetrolic acid (页面存档备份,存于)". Org. Synth. 42:97; Coll. Vol. 5:1043. - Coffman, Donald D. (1940). "Dimethylethynylcarbinol (页面存档备份,存于)". Org. Synth. 20:40; Coll. Vol. 3:320.
Hauser, C. R.; Adams, J. T.; Levine, R. (1948). "Diisovalerylmethane (页面存档备份,存于)". Org. Synth. 28:44; Coll. Vol. 3:291. - Vanderwerf, Calvin A.; Lemmerman, Leo V. (1948). "2-Allylcyclohexanone (页面存档备份,存于)". Org. Synth. 28:8; Coll. Vol. 3:44.
- Hauser, Charles R.; Dunnavant, W. R. (1960). "α,β-Diphenylpropionic acid (页面存档备份,存于)". Org. Synth. 40:38; Coll. Vol. 5:526.
Kaiser, Edwin M.; Kenyon, William G.; Hauser, Charles R. (1967). "Ethyl 2,4-diphenylbutanoate (页面存档备份,存于)". Org. Synth. 47:72; Coll. Vol. 5:559.
Wawzonek, Stanley; Smolin, Edwin M. (1951). "α,β-Diphenylcinnamonitrile (页面存档备份,存于)". Org. Synth. 31:52; Coll. Vol. 4:387. - Murphy, William S.; Hamrick, Phillip J.; Hauser, Charles R. (1968). "1,1-Diphenylpentane (页面存档备份,存于)". Org. Synth. 48:80; Coll. Vol. 5:523.
- Hampton, K. Gerald; Harris, Thomas M.; Hauser, Charles R. (1971). "Phenylation of diphenyliodonium chloride: 1-phenyl-2,4-pentanedione (页面存档备份,存于)". Org. Synth. 51:128; Coll. Vol. 6:928.
Hampton, K. Gerald; Harris, Thomas M.; Hauser, Charles R. (1967). - Potts, K. T.; Saxton, J. E. (1960). "1-Methylindole (页面存档备份,存于)". Org. Synth. 40:68; Coll. Vol. 5:769.
- Bunnett, J. F.; Brotherton, T. K.; Williamson, S. M. (1960). "N-β-Naphthylpiperidine (页面存档备份,存于)". Org. Synth. 40:74; Coll. Vol. 5:816.
- Brazen, W. R.; Hauser, C. R. (1954). "2-Methylbenzyldimethylamine (页面存档备份,存于)". Org. Synth. 34:61; Coll. Vol. 4:585.
- Allen, C. F. H.; VanAllen, J. (1944). "Phenylmethylglycidic ester (页面存档备份,存于)". Org. Synth. 24:82; Coll. Vol. 3:727.
- Allen, C. F. H.; VanAllen, James (1942). "2-Methylindole (页面存档备份,存于)". Org. Synth. 22:94; Coll. Vol. 3:597.
外部链接
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.